An Atlas of Lumps and Bumps, Part 29: Keratoacanthoma
Keratoacanthoma
Keratoacanthoma is an epithelial neoplasm characterized by a sharply demarcated dome-shaped nodule with a central keratin-filled crater, (Figure 1) rapid growth in the proliferative phase, variable period of tumor stability, and potential for spontaneous involution.1 The peak incidence is between 50 and 69 years of age.2,3 The male to female ratio is 2:1.2,3 The condition is most common in fair-skinned individuals.2

Figure 1. Keratoacanthoma is an epithelial neoplasm characterized by a sharply demarcated dome-shaped nodule with a central keratin-filled crater.
It is believed that keratoacanthoma derives from the infundibulum of the hair follicle.2 Changes in the expression of genes involved in epidermal cell proliferation, cell adhesion, and cell survival may play a pivotal role in the development of keratoacanthoma.2 In this regard, mutations of the tumor suppressor gene p53, H-Ras proto-oncogene, and the apoptosis regulatory protein gene bcl-2/Bak are implicated in the pathogenesis.2,3 Moreover, mutations in the transforming growth factor beta receptor and mismatch repair genes have been reported in multiple self-healing squamous epithelioma (MSSE, also known as multiple keratoacanthomas of Ferguson-Smith type, or Ferguson-Smith syndrome) and keratoacanthomas in Muir-Torre syndrome, respectively.4
Ultraviolet radiation is a major predisposing factor,1 and other predisposing factors include White individuals with Fitzpatrick skin phototypes I, II or III, genetic predisposition, local trauma or surgery, foreign bodies (collagen fillers, hyaluronic acid with acrylic fillers), immunodeficiency, medications (sorafenib, dabrafenib, vemurafenib, vismodegib), chemical carcinogens (pitch, tar, cigarette, polyaromatic hydrocarbons), and, possibly, certain types of human papillomavirus infection.2,5-7
According to the number, size, and distribution, several clinical variants of keratoacanthoma have been described, namely, solitary keratoacanthoma (vast majority), giant keratoacanthoma, subungual keratoacanthoma, mucosal keratoacanthoma, keratoacanthoma centrifugum marginatum, MSSE, generalized eruptive keratoacanthomas of Grzybowski, multiple keratoacanthomas of Witten and Zak, and eruptive squamous atypia (eruptive keratoacanthoma; Figure 2).2,7

Figure 2. A clinical variant of keratoacanthoma, eruptive keratoacanthoma.
Solitary keratoacanthoma is the most common variant.1,2 Typically, solitary keratoacanthoma presents as an asymptomatic, firm, solitary, pink, red or skin-colored, dome-shaped, berry shaped, or bud-shaped nodule with a central keratin-filled crater.2,7 Sites of predilections include sun-exposed, hair-bearing areas such as the face (Figure 3), neck, arms, and dorsal hands (Figure 4) as well as areas of previous trauma.1

Figure 3. Sites of predilections include sun-exposed, hair-bearing areas such as the face.
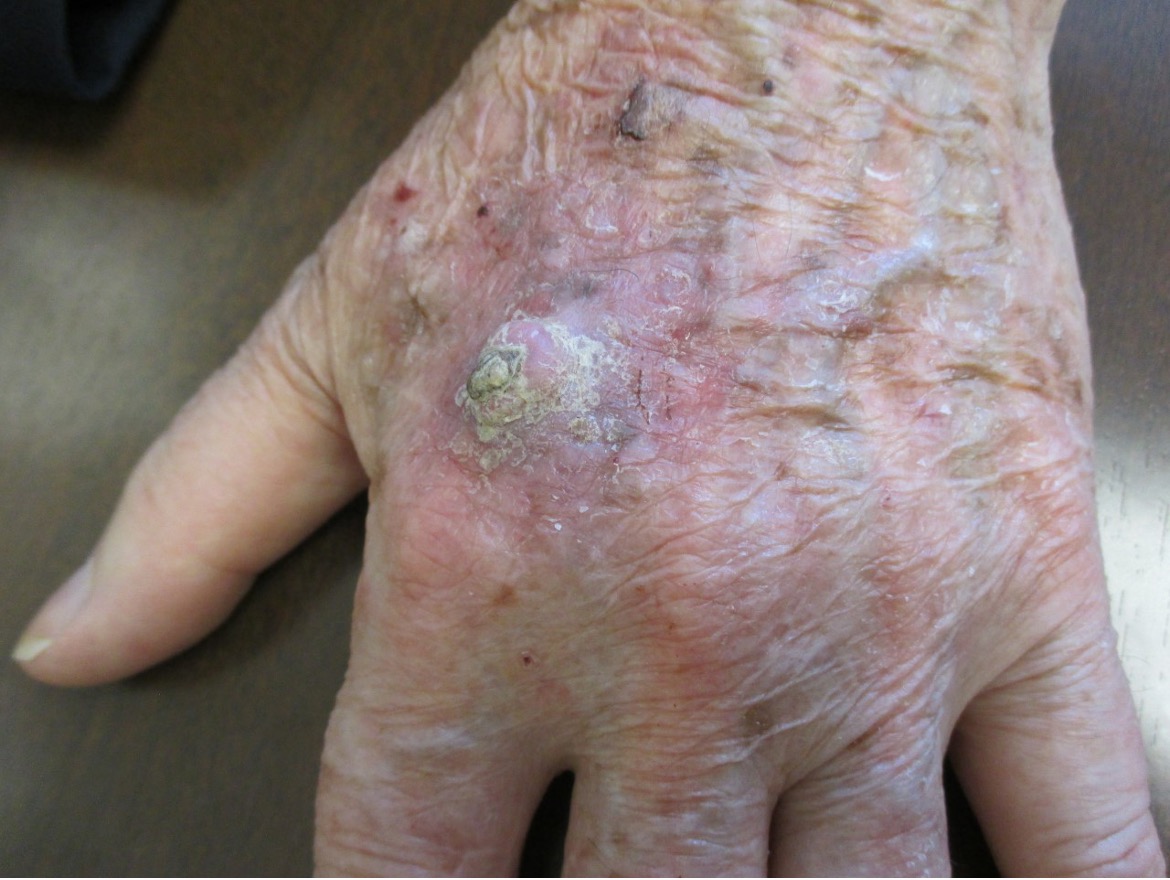
Figure 4. Sites of predilections include sun-exposed, hair-bearing areas such as the dorsal hands.
Solar-induced freckles, solar lentigines, and actinic keratoses may be found in the surrounding areas.1 The lesion is characterized by an initial period of rapid growth and achieves an average size of 1 to 2 cm in the first few weeks.3,7 It may be tender or sore. The lesion then stabilizes for several weeks to months and may regress spontaneously over several months, leaving an atrophic, often hypopigmented scar.2,3 Keratoacanthomas, especially those on the face and neck may, rarely, progress to invasive or metastatic carcinoma.3
Arbitrarily, a giant keratoacanthoma refers to a keratoacanthoma greater than 2 cm in diameter.8 Sites of predilection include the eyelids and nose.2 A giant keratoacanthoma can be locally invasive and destructive.1,9
A subungual keratoacanthoma develops on the nail bed and is usually painful.10 Typically, a subungual keratoacanthoma presents as a crescent-shaped soft tissue mass with erosion of the underlying bone. Sites of predilection include the thumbs, index fingers, and middle fingers.2 Although a subungual keratoacanthoma is locally aggressive, it does not metastasize.
Mucosal keratoacanthomas develop on mucosal surfaces such as the oral mucosa, lip, conjunctiva, nasal mucosa, and genitalia.11 Mucosal keratoacanthomas may also be seen in patients with generalized eruptive keratoacanthomas of Grzybowski.2
Keratoacanthoma centrifugum marginatum is characterized by progressive peripheral expansion with a raised rolled-out margin, central clearing, and atrophy.12,13 The most common locations are the face, trunk, and dorsa of hands and feet.2,13 Lesions are large, reaching to 20 cm or more.2,14 Keratoacanthoma centrifugum marginatum is differentiated from giant keratoacanthoma by prominent horizontal growth and absence of downward vertical growth and destruction of underlying tissue.13
MSSE is seen predominantly in Scottish kindreds. The condition has as an autosomal dominant mode of inheritance attributed to loss-of-function mutations in the transforming growth factor beta receptor 1 (TGFBR1 or ALK5) gene.2 MSSE often presents as multiple to hundreds of keratoacanthomas through adolescence and beyond.2
Generalized eruptive keratoacanthomas of Grzybowski is characterized by a sudden generalized eruption of numerous (may be hundreds to thousands), small (1 to 3 mm), follicular, skin- or flesh-colored papules with central umbilication that may contain a central horny, keratotic plug.15-17 The onset is usually between the fifth and seventh decades of life.2 The sex ratio is equal. The condition usually affects the skin and, less commonly, the mucous membrane.15,16 Sun-exposed areas are predominately affected with prominent facial involvement, leading to ectropion and a masked facies of tumors (sclerodermoid changes of the face) referred to as sign of Zorro.2,15 The palms and soles are usually spared. Intense pruritus is common.15
Multiple keratoacanthomas of Witten and Zak are characterized by multiple larger cherry-sized nodules, intermediate pea-sized lesions, and smaller follicular papules.1,14 The condition has as an autosomal dominant mode of inheritance. The age of onset is usually in childhood.
Eruptive squamous atypia (eruptive keratoacanthoma) refers to an idiopathic proliferation of well-differentiated squamous cell carcinoma with atypia.2
In addition, keratoacanthomas may be part of Muir-Torre syndrome, Ferguson-Smith syndrome, incontinentia pigmenti, and xeroderma pigmentosum.2,3,14
The diagnosis of keratoacanthoma is usually clinical, based on its distinctive clinical history and features. Dermoscopy of the lesion reveals keratin crust/scale, central keratin mass, white keratin pearls, white circles, white structureless zones, hemorrhage centrally and in areas of keratinization, glomerular vessels, linear irregular vessels, atypical vessels, and hairpin vessels.18,19 Unfortunately, these features may also be present in squamous cell carcinoma.18,19 Because of a lack of clinical features that can reliably distinguishing keratoacanthoma from squamous cell carcinoma, a biopsy with a sample of sufficient depth should be considered. Histological findings include epidermal hyperplasia, overhanging epithelial lips, central keratin-filled crater, keratinocytes with glassy eosinophilic cytoplasm, sharp demarcation between tumor nests and surrounding stroma, and mixed inflammatory infiltrate in the dermis.1,2,14 A keratoacanthoma does not extend into the dermis below the eccrine glands.14
AFFILIATIONS:
1Clinical Professor of Pediatrics, the University of Calgary, Calgary, Alberta, Canada
2Pediatric Consultant, the Alberta Children’s Hospital, Calgary, Alberta, Canada
3Dermatologist, Medical Director and Founder, the Toronto Dermatology Centre, Toronto, Ontario, Canada
4Associate Clinical Professor of Pediatrics, Dermatology and Skin Sciences, the University of British Columbia, Vancouver, British Columbia, Canada.
5Pediatric Dermatologist, the Pediatric Institute, Kuala Lumpur General Hospital, Kuala Lumpur, Malaysia
CITATION:
Leung AKC, Barankin B, Lam JM, Leong KF. An atlas of lumps and bumps, part 29: Keratoacanthoma. Consultant. 2023;63(7)e7. doi:10.25270/con.2023.06.000005
CORRESPONDENCE:
Alexander K. C. Leung, MD, #200, 233 16th Ave NW, Calgary, AB T2M 0H5, Canada (aleung@ucalgary.ca)
EDITOR’S NOTE:
This article is part of a series describing and differentiating dermatologic lumps and bumps. To access previously published articles in the series, visit https://www.consultant360.com/resource-center/atlas-lumps-and-bumps.
References
1. Leung AKC, Barankin B. How would you diagnose this woman’s growing asymptomatic nodule? Consultant. 2015;55:1042-1045.
2. Brewer JD. Keratoacanthoma: epidemiology, risk factors, and diagnosis. In: Post TW, ed. UpToDate. Waltham, MA. (Accessed on January 17, 2021)
3. Zito PM, Scharf R. Keratoacanthoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–2020 Sep 29. PMID: 29763106.
4. Savage JA, Maize JC Sr. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36(5):422-429. doi: 10.1097/DAD.0000000000000031.
5. Conforti C, Paolini F, Venuti A, Dianzani C, Zalaudek I. The detection rate of human papillomavirus in well-differentiated squamous cell carcinoma and keratoacanthoma: is there new evidence for a viral pathogenesis of keratoacanthoma? Br J Dermatol. 2019;181(6):1309-1311. doi: 10.1111/bjd.18212.
6. Kwiek B, Schwartz RA. Keratoacanthoma (KA): An update and review. J Am Acad Dermatol. 2016;74(6):1220-1233. doi: 10.1016/j.jaad.2015.11.033.
7. Ngo J, Barankin B. Keratoacanthoma. Can Fam Physician. 2008;54:985, 993. PMID: 18625820
8. Bogner PN, Cheney RT, Zeitouni NC. Giant keratoacanthoma: case report and review of the English literature. Am J Dermatopathol. 2014;36(3):252-257. doi: 10.1097/DAD.0b013e318291c582.
9. Pica Alfieri E, Sisti A, Nisi G, Brandi C, Grimaldi L, D'Aniello C. A giant keratoacanthoma of the cheek. Acta Biomed. 2019;90(4):580-582. doi: 10.23750/abm.v90i4.7409.
10. DE Vasconcelos P, Soares-Almeida L, Filipe P. Subungual keratoacanthoma in a pianist. G Ital Dermatol Venereol. 2016;151(4):455-6. PMID: 27348330.
11. AlBayyat GJ, Venkateswaran N, Arreaza D, Dubovy SR, Galor A, Karp CL. Spontaneous regression of conjunctival keratoacanthoma. BMJ Case Rep. 2019;12(7):e228833. doi: 10.1136/bcr-2018-228833.
12. Adya KA, Inamadar AC, Palit A. Dermoscopy of keratoacanthoma centrifugum marginatum. Indian Dermatol Online J. 2019;10(3):360-362. doi: 10.4103/idoj.IDOJ_134_18.
13. Phiske MM, Avhad G, Jerajani HR. Keratoacanthoma centrifugum marginatum at an unusual site. Indian J Dermatol. 2013;58(1):74-76. doi: 10.4103/0019-5154.105316.
14. Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28:254-261. doi: 10.1016/j.clindermatol.2009.06.010.
15. Anzalone CL, Cohen PR. Generalized eruptive keratoacanthomas of Grzybowski. Int J Dermatol. 2014;53:131-136. doi: 10.1111/ijd.12318.
16. Nofal A, Assaf M, Ghonemy S, Nofal E, Yosef A. Generalized eruptive keratoacanthomas: a diagnostic and therapeutic challenge. Int J Dermatol. 2015;54:160-167. doi: 10.1111/ijd.12308.
17. Rotola A, Musmeci D, Gentili V, Reale D, Borghi A, Rizzo R, et al. Generalized eruptive keratoacanthoma of the Grzybowski type: some considerations on treatment and pathogenesis. Int J Dermatol. 2019;58(12):e242-e245.
18. Lin MJ, Pan Y, Jalilian C, Kelly JW. Dermoscopic characteristics of nodular squamous cell carcinoma and keratoacanthoma. Dermatol Pract Concept. 2014;4(2):9-15. doi: 10.5826/dpc.0402a02.
19. Rosendahl C, Cameron A, Argenziano G, Zalaudek I, Tschandl P, Kittler H. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch Dermatol. 2012;148(12):1386-1392. doi: 10.1001/archdermatol.2012.2974.


