Peer Reviewed
Glioblastoma Multiforme
Authors:
Syed A. A. Rizvi, PhD, MS, MBA
Hampton University School of Pharmacy, Hampton, Virginia
Shazia Zafar, MD
Southwest Florida Cancer Care, Pembroke Pines, Florida
Sultan S. Ahmed, MD
University of Miami, Coral Gables, Florida
Nawal Zafar, BS
University of Miami, Coral Gables, Florida
Yasser Shahzad, PhD
COMSATS University Islamabad, Lahore Campus, Lahore, Pakistan
Ayman M. Saleh, PhD
King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
Jasmin Ahmed, MD
Larkin Community Hospital, Miami, Florida
Citation:
Rizvi SAA, Zafar S, Ahmed SS, Zafar N, Shahzad Y, Saleh AM, Ahmed J. Glioblastoma multiforme. Consultant. 2018;58(11):318-320.
A 52-year-old woman presented with a nonspecific headache of approximately 5 months’ duration, which had intensified within the past 30 days. The headache was associated with blurred vision bilaterally. She denied weight loss, aura, scintillating scotoma, dizziness, and neuromuscular disorders. She did not smoke, she had no known food or drug allergies, and her medical history was insignificant.
Physical examination. The patient was normal in appearance with no apparent cognitive deficiencies. Her blood pressure was 119/67 mm Hg, pulse was 59 beats/min, respiratory rate was 16 breaths/min, and body mass index was 38.89 kg/m2. Neuromuscular examination findings were normal.
Diagnostic tests. Sagittal T1-weighted magnetic resonance imaging (MRI) revealed a 3.7 × 3.0 × 3.3 cm, irregular, inhomogeneous mass in the posterior right frontal lobe with extensive surrounding vasogenic edema. Another mass in the left posteromedial temporal lobe straddling the left ventricular trigone was also identified, measuring 2.7 × 2.2 × 2.6 cm (Figure 1). The masses demonstrated irregular, thickened peripheral enhancement with a necrotic/cystic internal component and increased peripheral cerebral blood flow on the perfusion images.

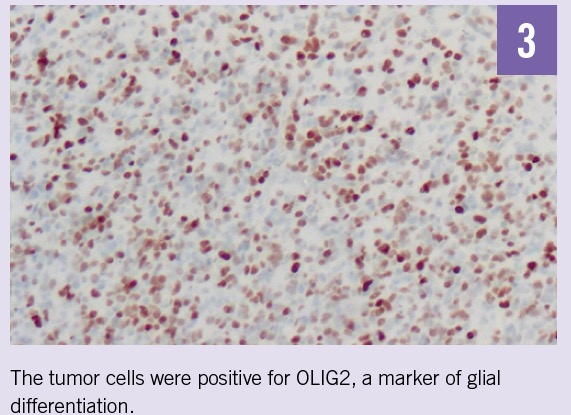
Routine hematoxylin-eosin sections demonstrated a malignant glial neoplasm of moderate to high cellularity composed predominantly of medium-sized cells with delicate fibrillary cytoplasm and oval, markedly hyperchromic nuclei with coarse chromatin and indistinct nucleoli (Figure 2). Necrosis and endothelial hyperplasia were present. The tumor cells were positive for glial fibrillary acidic protein (GFAP) and oligodendrocyte transcription factor (OLIG2) but negative for mutant IDH1-R132H protein, synaptophysin, and BRAF V600E mutation (Figures 3 and 4). Histological and molecular studies of the resected tumors confirmed the diagnosis of glioblastoma, IDH wild type, World Health Organization grade IV, positive for O6-methylguanine-DNA-methyltransferase (MGMT).


NEXT: Discussion
Discussion. Glioblastoma multiforme (GBM) is considered to be the most malignant and most common type of brain neoplasm, with an estimated incidence rate of 3 to 4 cases per 100,000 population per year and a 3-year postdiagnosis survival rate as low as 2.2%.1,2 It is an infiltrating tumor, thus complete surgical resection is often not possible.3,4 Therefore, tumor resection is followed by radiotherapy and chemotherapy, and even then GBM almost always relapses.5
MRI is the first diagnostic tool for brain tumors, followed by histopathological assessment to differentiate. Hallmarks of GBM include hypervascularization, nuclear pleomorphism, and necrotic foci, the latter being the most characteristic feature.6 Immunohistochemical markers include OLIG2, cyclin D1, GFAP, synaptophysin, Ki-67 nuclear marker, MGMT promotor methylation. and BRAF V600E mutation.7-11
The management of GBM starts by resecting as much tumor as achievable, followed by radiation and chemotherapy.12 Temozolomide (TMZ), an oral alkylating cytotoxic agent, is the drug of choice; however it has been noted that TMZ benefits patients mostly with methylated MGMT gene promotor.13 Thus, many new targeted therapies for GBM have been investigated, including angiogenesis inhibitors,14,15 integrins inhibitors,16 growth factor inhibitors,17 and immunotherapy.18
Outcome of the case. Our patient underwent resection of the bilateral temporal glioblastomas (Figure 5). Postoperatively, she received radiotherapy and TMZ. However, she did not tolerate TMZ well and is now receiving combination therapy with irinotecan and bevacizumab.

References:
- Hottinger AF, Stupp R, Homicsko K. Standards of care and novel approaches in the management of glioblastoma multiforme. Chin J Cancer. 2014;33(1):32-39.
- Smoll NR, Schaller K, Gautschi OP. The cure fraction of glioblastoma multiforme. Neuroepidemiology. 2012;39(1):63-69.
- Utsuki S, Oka H, Suzuki S, et al. Pathological and clinical features of cystic and noncystic glioblastomas. Brain Tumor Pathol. 2006;23(1):29-34.
- Walid MS. Prognostic factors for long-term survival after glioblastoma. Perm J. 2008;12(4):45-48.
- Park JK, Hodges T, Arko L, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28(24):3838-3843.
- Urbańska K, Sokołowska J, Szmidt M, Sysa P. Glioblastoma multiforme—an overview. Contemp Oncol (Pozn). 2014;18(5):307-312.
- Takahashi Y, Akahane T, Sawada T, et al. Adult classical glioblastoma with a BRAF V600E mutation. World J Surg Oncol. 2015;13:100.
- Zhang K, Wang X-q, Zhou B, Zhang L. The prognostic value of MGMT promoter methylation in glioblastoma multiforme: a meta-analysis. Fam Cancer. 2013;12(3):449-458.
- Matsumura N, Wang Y, Nakazato Y. Coexpression of glial and neuronal markers in the neurocytic rosettes of rosette-forming glioneuronal tumors. Brain Tumor Pathol. 2014;31(1):17-22.
- Christmann M, Nagel G, Horn S, et al. MGMT activity, promoter methylation and immunohistochemistry of pretreatment and recurrent malignant gliomas: a comparative study on astrocytoma and glioblastoma. Int J Cancer. 2010;127(9):2106-2118.
- Shivaprasad NV, Satish S, Ravishankar S, Vimalambike MG. Ki-67 immunostaining in astrocytomas: association with histopathological grade—a South Indian study. J Neurosci Rural Pract. 2016;7(4):510-514.
- Stupp R, Mason WP, van den Bent MJ, et al; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
- de Groot JF, Lamborn KR, Chang SM, et al. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2011;29(19):2689-2695.
- Rathinavelu A, Alhazzani K, Dhandayuthapani S, Kanagasabai T. Anti-cancer effects of F16: a novel vascular endothelial growth factor receptor-specific inhibitor. Tumour Biol. 2017;39(11): 1010428317726841.
- Gilbert MR, Kuhn J, Lamborn KR, et al. Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03-02, a phase II trial with measures of treatment delivery. J Neurooncol. 2012;106(1):147-153.
- Raizer JJ, Abrey LE, Lassman AB, et al; North American Brain Tumor Consortium. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010;12(1):95-103.
- Sampson JH, Aidape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13(3):324-333.


