Sleep Disruptions and Insomnia in Older Adults
ABSTRACT: Many older adults suffer from the inability to sleep. However, insomnia and disrupted sleep is a complex condition and should be evaluated by taking comorbidity, lifestyle, environment, and personal preferences into account. Physicians should generally rely on behavioral measures as the first step in therapy and factor in risk-benefit ratio before considering low-dose hypnotics and other medication. Management of sleep difficulties should allot for periodic reassessment to maximize benefits and minimize risks.
__________________________________________________________________________________________________________________________________________________
Changes in ability to sleep are common for older adults. Sleep architecture evolves with age, and may be associated with reduction in both total sleep time and the amount of time spent in deep or slow-wave sleep—as well as weakening of the suprachiasmatic nuclei (one’s biological clock) circadian rhythm. While old age is associated with an increased prevalence of insomnia symptoms, this correlation is not noticed in healthy older adults who slept well as when younger.1
Clinically, the most notable change in sleep is an increase in the number of nighttime awakenings and lower sleep efficiency (ratio of time in bed asleep to time in bed).2 This is likely due to changes in health and well-being—age, cognitive status, and medical burden appear to predict declines in sleep efficiency.3 In addition, sleep becomes increasingly disrupted with worsening of physical health,4 as well as symptoms of anxiety5 and depression.6
______________________________________________________________________________________________________________________________________________________
RELATED CONTENT
Toward a Good Night's Sleep: An Approach to Insomnia
Insomnia Inhibits Recovery From Depression
__________________________________________________________________________________________________________________________________________________________________
The Demographics
Approximately 6% of long-term care residents meet the diagnostic criteria for insomnia as compared to 4% of community-based older adults.7 Nursing home residents complain of difficulty falling and staying asleep, which in turn results in considerably lower sleep efficiency despite residents spending more time in bed than community-based samples.8
Older adults with dementia also face challenges in regard to sleep: Up to 50% of individuals with dementia experience decreased total sleep time, less slow-wave and rapid eye movement (REM) sleep, and frequent nocturnal awakenings7, which may be associated with confusional arousals, agitation, and wandering. Comorbid illnesses and primary sleep disorders can also contribute significantly to sleep disregulation. Medication used to treat dementia can contribute to sleep disruption: Donepezil may increase REM sleep, decrease REM sleep latency, or increase nightmares, while galantamine may decrease REM sleep latency and slow-wave sleep.
Finally, caregivers (usually a spouse or relative) for an individual with dementia also form a comprised sleep group. The caregivers often work in an overburdened schedule, similar to a rotating shift worker, but without much predictability. Difficulties with sleep in these circumstances (ie, nighttime awakenings, shorter sleep time, and increased wake time after sleep onset) are very common.9 The quality of the caregiver’s sleep is related to the degree of cognitive impairment and nighttime activity of the person being cared for, as well as the caregiver’s age, and mental and physical health. This is one of the common factors in deciding to institutionalize the affected individual.10
Adverse Health Effects
Sleep and its disruption has been the object of increased investigation due to its clinical, psychosocial, and therapeutic importance. Insomnia has been linked to daytime sleepiness, diminished cognitive ability (including attention and memory), disturbances in psychomotor functioning, and slowed response time.
In a longitudinal study of adults ages 65 years and older who were cognitively intact at baseline, chronic symptoms of insomnia were found to be a significant and independent risk factor for cognitive decline in men without depression.11 Other impairments noted to be associated with insomnia include inability to enjoy family and social relationships, increased incidence of pain, decreased ability to accomplish daily tasks, poor self-rated health, and increased consumption of healthcare resources.12 Insomnia has been shown to be associated with poorer psychological and social well-being—leading to a lower quality of life.13 Particularly in males, insomnia was found to be the strongest predictor of both mortality and long-term care placement.14
Evaluation
The etiology of insomnia and disrupted sleep in older adults is often juxtaposed against other health conditions, otherwise known as comorbid insomnia. Furthermore, comorbid insomnia can be differentiated based on whether an accompanying condition plays a role in the insomnia, is the consequence of insomnia, or is incidental to insomnia. Approximately half of all insomnia subjects have been reported to have long-term medical problems.15
Prior management of insomnia indicated that if insomnia occurred within the context of comorbidity, it should become the focus of treatment. However, this may not account for the more complex nature of insomnia. The “3 P’s” of insomnia include medical conditions or other sleep disruptors acting as precipitating (and even perpetuating) events, predisposing conditions (eg, high trait anxiety) and perpetuating factors (eg, inactivity and excessive napping during the day). In addition, most cases of insomnia among older adults likely include multiple precipitating and predisposing factors, both physiological and behavioral. The Figure illustrates the multifactorial nature of comorbid insomnia.

Common Contributors to Insomnia
Medical conditions, psychiatric illnesses, sleep disorders, and the medications to treat them, all contribute to age-related changes in sleep architecture and circadian rhythm changes. Host factors include an increased tendency to hyperarousal, a personality prone to worry, “lark versus owl” disposition, and contribute to insomnia pathophysiology. Environmental factors and poor sleep habits can perpetuate insomnia.
•Pain. A common contributor to insomnia, painful conditions are frequently associated with reduced total sleep time, reduced REM sleep, frequent brief arousals and increased wakefulness after sleep onset. In a population of adults ages 55 to 84, 19% reported that pain disrupted their sleep at least a few nights per week and 12% reported almost nightly sleep fragmentation.6
In referral populations of patients with chronic pain, prevalence rates of insomnia can range between 50% to 70%.16 Another study of adults over age of 65 with knee arthritis were observed to have problems initiating sleep (31%), maintaining sleep (81%), and a tendency to awaken early in the morning (51%).17 Insomnia also has a >75% prevalence in fibromyalgia patients.18
•Gastroesophageal reflux disease (GERD). In population surveys, between 50% to 70% of individuals with GERD report nighttime symptoms and reduced sleep quality.19 In a sample of 11,685 survey respondents with GERD, 89% experienced nighttime symptoms, 68% sleep difficulties, 49% difficulty initiating asleep, and 58% difficulty maintaining sleep.20
•Hypertension and heart disease. In a sample of patients with hypertension, almost 50% were identified as having insomnia—which is more frequently seen in women (61%) and coronary artery disease (CAD) patients.21 In stable CAD patients, 42% of women reported “too little sleep” as compared to 24% of men.22
In patients with congestive heart failure (CHF), consistent difficulties maintaining sleep were reported by approximately 20% of sample, while 25% of the subjects were awake up to 3 hours/night.23 In another sample of CHF patients, 31% reported symptoms suggestive of chronic insomnia; paroxysmal nocturnal dyspnea (PND) had an odds ratio of 3.5 for a complaint of insomnia.24
•Chronic obstructive lung disease. This condition increases sleep stage changes, decreases total sleep time, and increases number of arousals.25 Patients are impacted by nocturnal cough, wheezing, and shortness of breath due to worsening of pulmonary mechanics and gas exchange during sleep. Hypoxia, which may occur particularly during REM sleep, has been correlated with an increase in arousal and excessive daytime sleepiness.
•Nocturnal asthma. These symptoms result in nighttime awakenings that occur in over 70% of persons with asthma. In a large sample of asthmatic patients who maintained a record of acute asthma episodes, asthma was approximately 70-fold more frequent between 4 am and 5 am than between 2 pm and 3 pm.26 Difficulties in initiating sleep, maintaining sleep, and nocturnal wakefulness appear to correlate with the number of nocturnal breathing problems.27
•Urologic and chronic renal diseases. The incidence and severity of nocturia increase with age. In older adults, prevalence of nocturia (≥1 void) reaches 75% of patients. Nocturia was listed as a self-perceived cause of disrupted nocturnal sleep “every night or almost every night” by half of a sample of subjects ages 55 to 84 years.28
While urinary incontinence, recurrent cystitis and diabetes mellitus were found to be strongest risk factors for nocturia,29 other factors include polyuria, low bladder capacity, sleep apnea, intake of excessive fluid before bedtime, alcohol, caffeine, diuretics, as well as medical disorders such as hypertension, CHF, and prostatic disease.30
More than half of patients with end-stage renal disease report sleep maintenance problems and early morning awakening.31 Quality of sleep was found to be associated with hemoglobin and serum albumin levels and depression.32 Conversely, the treatment of anemia with erythropoietin improves sleep quality.33
•Cancer. Patients with cancer may suffer from insomnia due to the disease itself, its treatment, and psychological responses to the diagnosis.34 Epidemiological studies reported sleep problems being present in up to 70% of patients.35 Approximately half of affected patients report onset within 6 months prediagnosis to 18 months postdiagnosis.36 Difficulties with sleep remained present in almost 25% of patients at the 1-year follow-up.37
•Neurologic disorders. Different neurologic disorders, including Parkinson’s disease (PD) and Alzheimer's disease (AD), are associated with a high prevalence of insomnia. In PD, insomnia may be the result of motor symptoms, psychiatric symptoms, and/or treatment with dopaminergic agents.38 Difficulty in maintaining sleep is particularly burdensome in patients with AD, and both insomnia and nocturnal agitation/sleep schedule disruption (eg, sundowning) is present in as many as 25% of patients and often leading to institutionalization.39
•Depressive disorders. Insomnia in the primary care setting shows a stronger association to depression than any other medical disorder.40 Approximately 75% of depressed adults and older adults complain of difficulty falling and staying asleep or being tired during the day. These subjective sleep complaints may persist beyond the resolution of depressive symptoms.41 Insomnia was shown to be a strong risk factor for future depression. Insomnia at baseline and 1 year later increased 8 times the risk of development of depression.42
•Anxiety disorders. Sleep disturbance is reported by two-thirds of patients with anxiety. Generalized anxiety disorder—a condition of generalized hyper arousal—is the most prevalent condition in patients complaining of insomnia who have a mental health diagnosis in the adult population.43 Patients report frequent nocturnal awakenings, prolonged time to fall asleep, decreased sleep efficiency and time, inconsistent findings of more stage 2 and decreased slow wave sleep.44
•Bereavement and grief. Insomnia was reported by 13% of subjects between 4 and 60 months (average 26 months) from the loss event.45 Baseline grief scores of widowed individuals was reported to be associated with sleep difficulties at the 18-month follow-up.46 Higher grief tended to be associated with less time spent asleep and reduced alertness at 8 pm.47
•Suboptimal sleep habits and circadian rhythm changes. Daytime sleep, if excessive, may interfere with participation in daytime activities and socialization, and increase the likelihood of nighttime awakenings. A significant part of the day may be spent “dozing,” to the point of daytime sleep becoming equal in duration to that of nighttime sleep. This is especially prevalent in institutionalized older adults or those with dementia.
With more medical illnesses and impairments, many older adults spend longer time in bed in their rooms and rarely go outdoors where they would be exposed to natural light. Severity of dementia in an individual has been correlated with levels of light exposure: The more severe the dementia, the less amount of light received.
Community-dwelling elderly persons with mild dementia were reported to receive less than 30 minutes of bright light exposure per day, while for a long-term care resident, natural light exposure can be as low as 10 minutes per day.48 Diminished light exposure does influence the circadian rhythm regulation, resulting in early-morning awakening and fragmented sleep. Other environmental factors that have an effect on circadian rhythms, such as physical activity and regular social interaction, are often deficient as well.13
A thorough evaluation is required to determine the specific factors contributing to insomnia in each older adult. To identify important contributors, obtain a complete sleep history, including a review of ongoing and past medical conditions, psychiatric illnesses, and a review of past and current medications. A physical examination and laboratory evaluation as indicated from the history are recommended to support the history and active health problems.
(Next Page: Tools for Assessing Sleep Symptoms)
Tools for Assessing Sleep Symptoms
A diagnosis of insomnia is made from patient’s own reports of reduced ability to sleep, sleep disruption, or unrefreshing nature of sleep associated with daytime consequences. While sleep polysomnography is an objective method that has been used in research for measuring sleep initiation and maintenance variables, difficulties with sleep are not currently considered an indication for clinical polysomnography unless a sleep disorder, such as sleep apnea or periodic limb movement disorder, is suspected.
•Sleep diary. A sleep diary is a form used by patients for recording sleep/wake schedules. It can vary in complexity from a simple log marking bedtime, nighttime awakenings, and morning wake times to a more detailed diary of daytime activities that may influence sleep. Sleep diaries should be completed for at least 1 to 2 weeks due to night-to-night variability. Once a baseline sleep pattern is established, sleep diaries can be used by the patient and provider to monitor treatment progress and symptom reoccurrence.
•Insomnia Severity Index. This questionnaire used to assess the severity of insomnia, satisfaction with current sleep, sleep interference, and concerns with the sleeping problem. There are 7 items and scores range from 0 to 28, with scores greater than 8 suggestive of insomnia.49
•Pittsburgh Sleep Quality Index. This survey measures the quality and patterns of sleep and differentiates “poor” from “good” sleep by measuring 7 areas: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction over the previous month. The patient self-rates each of these 7 areas of sleep: a sum of 5 or greater indicated a “poor” sleeper and resulted in a sensitivity of nearly 99% and specificity of 84% as a marker for sleep disturbances in patients with insomnia versus controls.50
•Epworth Sleepiness Scale. This is a self-estimate of sleep propensity in different circumstances and is commonly used to estimate the degree of sleepiness.51 Patients rate how likely they are to doze off or fall asleep in 8 different situations commonly encountered in daily life. Scores higher than 10 suggest excessive daytime sleepiness.
•Caregiver reports. When the use of self-assessment tools, such as sleep diaries and self-report sleep questionnaires, are not possible (eg, persons with severe cognitive impairment), caregiver reports are referenced. Note: There are major limitations when someone other than the patient is reporting the symptoms.
•Actigraphy. An accelerometer usually worn at the wrist, this tool provides valuable data in evaluating activity-wake patterns in long-term care settings due to major limitations in obtaining self-reported data, as well as limited feasibility of polysomnographic testing in this population.28 Actigraphy is usually employed for a period of 7 to 14 days and may assist with the differential diagnosis, guide treatment recommendations, and assess results. Actigraphy should not be used alone, but rather in conjunction with a sleep diary to guide scoring and interpretation of data.
_____________________________________________________________________________________________________________________________________________________
RELATED CONTENT
Poor Sleep Habits Put the Obese Teen at Risk
Non-Restorative Sleep Linked to Widespread Pain
_____________________________________________________________________________________________________________________________________________________
Management of Insomnia
Insomnia in older adults tends to be chronic and recurring,52 so long-term disease management strategies are needed. Medications alone may not be effective, can lose effectiveness over time, or even lead to increased risk of side effects in higher doses. Since medical and psychiatric conditions have a significant influence on sleep, optimizing management of underlying disorders should be part of the first step to improve sleep disruption. For example, controlling nocturnal symptoms disrupting sleep and optimizing control of comorbidities may improve difficulties with sleep. Assessing and managing sleep disorders are also part of this step. Since medications can increase arousal, contribute to sleepiness, or negatively influence the circadian rhythm, modifications in the timing of medications (eg, antidepressants) may also enhance sleep.
•Pharmacologic agents. While use of sedatives is not encouraged, use of short-acting, nonbenzodiazepine (BZD) agents may be advocated in conjunction with behavioral therapies to optimize treatment effectiveness of both.49 Hypnotics can decrease time to fall asleep and wake time during the night, but there are concerns about extended use in the older adults due to risk of side effects.
Approximately 30% of older adults who receive a new prescription for BZDs reported at least daily use 2 months later.53 It has been suggested that once individuals experience success with behavioral strategies, they may gain the confidence to comfortably taper hypnotic use without relapse. Discontinuation of BZD treatment for insomnia can be a difficult task. In a sample of older adults followed for a 2-year period, approximately 43% of the individuals had resumed BZD use.54 Predictors of relapse include insomnia severity, psychological distress, and number of drug-free weeks.
•Cognitive behavioral therapy. In younger population, cognitive behavioral strategies have been reported to be most effective in managing insomnia over time.55 Cognitive behavioral therapy for insomnia (CBT-I) is a multidimensional approach that combines psychological and behavioral therapies to treat insomnia. These include sleep hygiene, sleep restriction, stimulus control, relaxation techniques, and cognitive therapy. Any single therapy does not appear as effective as when interventions are combined.56
In patients with chronic sleep difficulties, long-term hypnotic consumption, and high levels of comorbidity, use of CBT-I has been reported to improve sleep latency, sleep efficiency, and global sleep quality,57 with most improvements in sleep quality remaining present at the 3 to 6 month follow-up.
Older age by itself was not found to be a barrier to successful treatment outcomes in using cognitive behavioral therapy. Efficacy coupled with minimal side effects makes behavioral interventions highly recommended for treating insomnia. However, most data relies on research studies employing highly specialized personnel such as psychologists, many of which are trained in behavioral sleep medicine. Factors such as cost, lack of availability of behavioral sleep medicine specialists, and potential problems with patient cognitive status, physical limitations, motivation, and compliance may raise challenges in implementing the use of CBT-I techniques.58

Lower health status at baseline, acute illness episodes, and hospitalizations may further lower success rates.57
•Sleep hygiene. Sleep hygiene measures are often the first form of intervention used to treat insomnia (Table 1). Sleep hygiene uses a generic package of recommendations to promote sleep, such as reducing environmental factors (ie, turning off TV) and intrinsic factors (ie, restricting alerting stimuli prior to bedtime) that can disrupt sleep. Sleep hygiene has been linked to sleep practices and, in turn, to overall sleep quality.
•Sleep restriction. Sleep restriction utilizes an individual’s natural circadian rhythm and homeostatic sleep drive to regulate sleep and wake patterns (Table 2).59 Sleep restriction interventions focus on limiting time in bed and daytime napping, and warding off unintentional dozing. Usually based on sleep diaries kept for 2 weeks prior to the intervention, the amount of time spent in bed is reduced to an amount closer to the estimated actual time sleeping. Once sleep efficiency (total sleep time divided by time spent in bed) improves, the time allowed in bed may be gradually increased in small increments until the individual’s optimal sleep time is obtained or sleep efficiency falls.
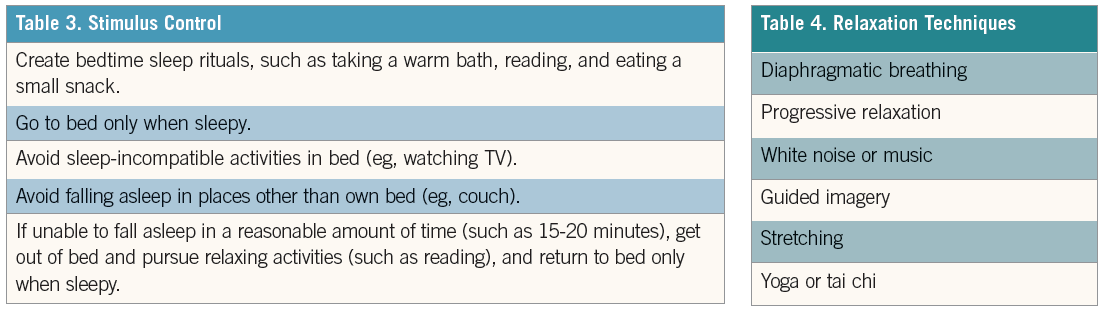
•Stimulus control. Stimulus control interventions focus on reducing conditioned behaviors that may interfere with sleep (Table 3).60 This may target patients who spend extended hours in bed trying to sleep, watching TV, or doing other activities, conditioning the body to use the bed as a place for activity instead of sleep.
•Relaxation. Relaxation techniques are used to create a relaxation response, help manage anxiety, and prepare the body for sleep (Table 4). These interventions are best suited for individuals who have problems with hyperarousal when trying to fall asleep.
•Cognitive interventions. Cognitive interventions address maladaptive sleep-related beliefs or intrusive presleep cognitions and generally require the assistance of a person trained in behavioral sleep interventions.
•Circadian rhythm modulators. The circadian component of sleep physiology can dampen with age, but can be also significantly impacted by neurologic conditions affecting the circadian centers (suprachiasmatic nuclei) and maladaptive behaviors (poor timing or suboptimal exposure to zeitgebers, such as light, meals, activity, and social interactions).
Light therapy may be attempted to reset the phase of the circadian rhythm relative to the light-dark cycle and also may influence the production of melatonin, which helps synchronize an individual’s circadian rhythm (Table 5). Bright light poses a theoretical threat of retinal damage in susceptible individuals (proliferative diabetic retinopathy, moderate or severe macular degeneration, or absence of a natural or artificial lens in either eye). While bright white light (similar to daylight) was initially employed, short-wavelength blue light (approximately 460 nm) may have greater phase-shifting properties than the rest of the visible light spectrum; it’s still unclear whether this is becoming the preferred method to influence the biological clock in the elderly.61
Endogenous nocturnal melatonin, which is a major loop for the circadian rhythm, may be decreased in older adults. Administration of melatonin 1 to 2 hours before bedtime may replicate the natural secretion pattern of melatonin, thereby leading to improvements in the circadian regulation of the sleep-wake cycle. In patients with primary insomnia aged ≥55 years, use of melatonin 1 to 2 hours before bedtime was associated with improvements relative to placebo in sleep quality and latency, morning alertness, and health-related quality of life.62

•Physical activity. A useful adjunctive therapy, physical activity improves total sleep duration, sleep-onset latency, and scores on a scale of global sleep quality.63 Benefits may extend beyond general physical activity—eg, a program of tai chi improved sleep-onset latency with 18 minutes and sleep duration with 48 minutes as compared with low-impact exercise participants.64
•Daily routine. There is emerging evidence that a consistent and structured daily routine may have beneficial effects on the quality of nighttime sleep. Research suggests that subjects with higher levels of lifestyle regularity report fewer sleep problems. Increased stability in daily routine was recently reported to predict shorter sleep latency and improved sleep quality—independent of functional status, comorbidities, and age.65 Therefore, interventions designed at regularizing patients’ daily rhythms and maintenance of a social rhythm would probably be beneficial.66
Due to complex landscape of insomnia and disrupted sleep in older adults, many factors including comorbidity, lifestyle, environment, and personal preferences as well as increased risks with sedative agents must be considered. Behavioral measures should be considered as the first line of therapy for the long-term control of insomnia. These may provide the best risk-benefit ratio while increasing the ability to sparingly use hypnotics or low-dose medication. Taking a long view of the insomnia management would require a multicomponent strategy employing carefully considered interventions and goals of therapy, while allowing for periodic reassessments and realignment of therapy to maximize benefits and minimize risks. ■
(Next Page: Tailoring Behavioral Interventions and References)
Tailoring Behavioral Interventions
• Long-term care. Differences in environment, health, and level of independence in individuals in long-term care settings may argue for a distinct approach to CBT-I interventions as compared to those used in community-based older adults. Behavioral interventions to improve sleep in long-term care settings must be tailored to the specific needs of each resident, and therapies should be chosen to target behaviors that are most likely to impact the individual resident’s sleep problems. Even when used alone, certain interventions have resulted in positive outcomes with continuation and improvement of sleep patterns for at least 2 years.52
•Dementia. Because of frequently undesirable side effects of pharmacologic agents in individuals with dementia, CBT-I would be an ideal intervention to implement; however, there are limitations to address when attempting cognitive interventions in persons with cognitive impairments.
Therefore, behavioral and environmental interventions appear most feasible and have to be tailored to the patient, included reducing day in-bed time, increasing social and physical activity, and altering the environment to make it more conducive to nighttime sleep. The Nighttime Insomnia Treatment and Education for Alzheimer's Disease (NITE-AD) program involved an 8-week combined intervention that included sleep hygiene education, daily walking (30 minutes), and increased light exposure (light box used for 1 hour per day), which reduced the time spent awake during the night by 32% compared with controls, with effects maintained at 6-month follow-up.67 Nonetheless, interventions that require changing established bedtime and waking routines and keeping a person with dementia awake during the day can be challenging for family caregivers and institutions to achieve.68
_____________________________________________________________________________________________________________________________________________________
RELATED CONTENT
Study Ties Troubled Sleep to Lower Brain Volume
Sleep Apnea Index Misses CV Warning Signs
_____________________________________________________________________________________________________________________________________________________
Mihai Teodorescu, MD, is an assistant professor of geriatrics and sleep medicine at the University of Wisconsin-Madison and the William S. Middleton Veteran Affairs Medical Center, Madison, WI.
References:
1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97-111.
2.Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
3.Hoch CC, Dew MA, Reynolds CF, 3rd, et al. A longitudinal study of laboratory- and diary-based sleep measures in healthy "old old" and "young old" volunteers. Sleep. 1994;17:489-496.
4.Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002; 53:555-559.
5.Maggi S, Langlois JA, Minicuci N, et al. Sleep complaints in community-dwelling older persons: prevalence, associated factors, and reported causes. J Am Geriatr Soc. 1998;46:161-168.
6.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497-502.
7.Voyer P, Verreault R, Mengue PN, et al. Prevalence of insomnia and its associated factors in elderly long-term care residents. Arch Gerontol Geriatr. 2006;42:1-20.
8.Ancoli-Israel S, Parker L, Sinaee R, et al. Sleep fragmentation in patients from a nursing home.
J Gerontol. 1989;44:M18-21.
9.Beaudreau SA, Spira AP, Gray HL, et al. The relationship between objectively measured sleep disturbance and dementia family caregiver distress and burden. J Geriatr Psychiatry Neurol. 2008;21:159-165.
10.Hope T, Keene J, Gedling K, et al. Predictors of institutionalization for people with dementia living at home with a carer. Int J Geriatr Psychiatry. 1998;13:682-690.
11.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185-1189.
12.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005; 53:S264-271.
13.Hidalgo JL, Gras CB, Garcia YD, et al. Functional status in the elderly with insomnia. Qual Life Res. 2007;16(2):279-86.
14.Pollak CP, Perlick D, Linsner JP, et al. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health. 1990;15:123-135.
15.Bixler EO, Kales A, Soldatos CR, et al. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1257-1262.
16Latham J, Davis BD. The socioeconomic impact of chronic pain. Disabil Rehabil. 1994; 16:39-44.
17.Wilcox S, Brenes GA, Levine D, et al. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48:1241-1251.
18.Ancoli-Israel S. The impact and prevalence of chronic insomnia and other sleep disturbances associated with chronic illness. Am J Manag Care. 2006;12:S221-229.
19.Farup C, Kleinman L, Sloan S, et al. The impact of nocturnal symptoms associated with gastroesophageal reflux disease on health-related quality of life. Arch Intern Med. 2001;161:45-52.
20.Mody R, Bolge SC, Kannan H, et al. Effects of gastroesophageal reflux disease on sleep and outcomes. Clin Gastroenterol Hepatol. 2009; 7:953-959.
21.Prejbisz A, Kabat M, Januszewicz A, et al. Characterization of insomnia in patients with essential hypertension. Blood Press. 2006;15:213-219.
22.Edell-Gustafsson U, Svanborg E, Swahn E. A gender perspective on sleeplessness behavior, effects of sleep loss, and coping resources in patients with stable coronary artery disease. Heart Lung. 2006;35:75-89.
23.Brostrom A, Stromberg A, Dahlstrom U, et al. Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs. 2004;19:234-242.
24.Principe-Rodriguez K, Strohl KP, Hadziefendic S, et al. Sleep symptoms and clinical markers of illness in patients with heart failure. Sleep Breath. 2005; 9:127-133.
25.Fleetham J, West P, Mezon B, et al. Sleep, arousals, and oxygen desaturation in chronic obstructive pulmonary disease. The effect of oxygen therapy. Am Rev Respir Dis. 1982;126:429-433.
26.Dethlefsen U, Repgas R. Ein neues Therapieprinzip bei Nachtlichen Asthma. Klin Med. 1985;80:44-47.
27.Janson C, Gislason T, Boman G, et al. Sleep disturbances in patients with asthma. Respir Med. 1990; 84:37-42.
28.Bliwise DL, Foley DJ, Vitiello MV, et al. Nocturia and disturbed sleep in the elderly. Sleep Med. 2009; 10:540-548.
29.Bing MH, Moller LA, Jennum P, et al. Nocturia and associated morbidity in a Danish population of men and women aged 60-80 years. BJU Int. 2008;
102(7):808-14.
30.Asplund R, Aberg H. Diurnal variation in the levels of antidiuretic hormone in the elderly. J Intern Med. 1991;229:131-134.
31.Williams SW, Tell GS, Zheng B, et al. Correlates of sleep behavior among hemodialysis patients. The kidney outcomes prediction and evaluation (KOPE) study. Am J Nephrol. 2002;22:18-28.
32.Iliescu EA, Coo H, McMurray MH, et al. Quality of sleep and health-related quality of life in haemodialysis patients. Nephrol Dial Transplant. 2003; 18:126-132.
33.Benz RL, Pressman MR, Hovick ET, et al. A preliminary study of the effects of correction of anemia with recombinant human erythropoietin therapy on sleep, sleep disorders, and daytime sleepiness in hemodialysis patients (The SLEEPO study). Am J Kidney Dis. 1999;34:1089-1095.
34.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895-908.
35.Savard J, Ivers H, Villa J, et al. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29:3580-3586.
36.Davidson JR, MacLean AW, Brundage MD, et al. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54:1309-1321.
37.Kozachik SL, Bandeen-Roche K. Predictors of patterns of pain, fatigue, and insomnia during the first year after a cancer diagnosis in the elderly. Cancer Nurs. 2008;31:334-344.
38.Thorpy MJ. Sleep disorders in Parkinson's disease. Clin Cornerstone. 2004;6 Suppl 1A:S7-15.
39.Bliwise DL. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone. 2004;6 Suppl 1A:S16-28.
40.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002; 51:229-235.
41.Reynolds CF, 3rd, Hoch CC, Buysse DJ, et al. Sleep in late-life recurrent depression. Changes during early continuation therapy with nortriptyline. Neuropsychopharmacology. 1991;5:85-96.
42.Roberts RE, Shema SJ, Kaplan GA, et al. Sleep complaints and depression in an aging cohort: A prospective perspective. Am J Psychiatry. 2000; 157:81-88.
43.Monti JM, Monti D. Sleep disturbance in generalized anxiety disorder and its treatment. Sleep Med Rev. 2000;4:263-276.
44.Benca RM, Obermeyer WH, Thisted RA, et al. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651-668; discussion 669-670.
45.Kowalski SD, Bondmass MD. Physiological and psychological symptoms of grief in widows. Res Nurs Health. 2008;31:23-30.
46.Prigerson HG, Frank E, Kasl SV, et al. Complicated grief and bereavement-related depression as distinct disorders: preliminary empirical validation in elderly bereaved spouses. Am J Psychiatry. 1995; 152:22-30.
47.Monk TH, Begley AE, Billy BD, et al. Sleep and circadian rhythms in spousally bereaved seniors. Chronobiol Int. 2008;25:83-98.
48.Martin JL, Webber AP, Alam T, et al. Daytime sleeping, sleep disturbance, and circadian rhythms in the nursing home. Am J Geriatr Psychiatry. 2006; 14:121-129.
49.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
50.Backhaus J, Junghanns K, Broocks A, et al. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737-740.
51.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545.
52.Reynolds CF, 3rd, Buysse DJ, Kupfer DJ. Treating insomnia in older adults: taking a long-term view. JAMA. 1999;281:1034-1035.
53.Simon GE, Ludman EJ. Outcome of new benzodiazepine prescriptions to older adults in primary care. Gen Hosp Psychiatry. 2006;28:374-378.
54.Morin CM, Belanger L, Bastien C, et al. Long-term outcome after discontinuation of benzodiazepines for insomnia: a survival analysis of relapse. Behav Res Ther. 2005;43:1-14.
55.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994; 151:1172-1180.
56.Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998-2004). Sleep. 2006; 29:1398-1414.
57.Morgan K, Dixon S, Mathers N, et al. Psychological treatment for insomnia in the management of long-term hypnotic drug use: a pragmatic randomised controlled trial. Br J Gen Pract. 2003;53:923-928.
58.Benca RM. Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv. 2005;56:332-343.
59.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45-56.
60.Bootzin RR, Perlis ML. Nonpharmacologic treatments of insomnia. J Clin Psychiatry. 1992;53 Suppl:37-41.
61.Herljevic M, Middleton B, Thapan K, et al. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40:237-242.
62.Lyseng-Williamson KA. Melatonin prolonged release: in the treatment of insomnia in patients aged >/=55 years. Drugs Aging. 2012;29:911-923.
63.Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002:CD003404.
64.Li F, Fisher KJ, Harmer P, et al. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geriatr Soc. 2004;52:892-900.
65.Zisberg A, Gur-Yaish N, Shochat T. Contribution of routine to sleep quality in community elderly. Sleep. 2010;33:509-514.
66.Monk TH, Reynolds CF, 3rd, Buysse DJ, et al. The relationship between lifestyle regularity and subjective sleep quality. Chronobiol Int. 2003;20:97-107.
67.McCurry SM, Gibbons LE, Logsdon RG, et al. Nighttime insomnia treatment and education for Alzheimer's disease: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:793-802.
68.Deschenes CL, McCurry SM. Current treatments for sleep disturbances in individuals with dementia. Curr Psychiatry Rep. 2009;11:20-26.


