Peer Reviewed
Screening and Management of Prostate Cancer in the Elderly
Prostate cancer is the most prevalent malignancy in elderly men and is the second leading cause of cancer-related death in the United States.1 Men >65 years of age will compose 19.6% of the population by 2030 compared with 12.4% in 2000, translating to a 3-fold population increase in the next 20 years. As a result, the incidence and prevalence of prostate cancer and of men who require treatment will continue to increase in the upcoming years. The optimum strategy for the screening and management of prostate cancer in the elderly population is a matter of considerable importance to the medical community at large and specifically to those who deliver medical care to the elderly. This article reviews the relevant issues surrounding the screening and management of prostate cancer in the elderly.
Epidemiology
Prostate cancer is a disease of elderly men. The SEER (Surveillance Epidemiology and End Results) Cancer Statistics Review estimated 217,730 new cases of prostate cancer and 32,050 prostate cancer deaths in 2010.1 From 2004 to 2008, the median patient age at prostate cancer diagnosis was 67 years, with 35.3% of patients receiving the diagnosis between the ages of 65 and 74 years, 19.9% between 75 and 84 years, and 4.4% at ≥85 years.2 From 2003 to 2007, the median age at death for patients with prostate cancer was 80 years, with 19.9% of deaths occurring in those between the ages of 65 and 74 years, 40.3% between 75 and 84 years, and 30.8% at ≥85 years.1 The lifetime risk of developing prostate cancer is 16.22%.1
The introduction of prostate cancer screening with serum prostate-specific antigen (PSA) has led to prostate cancer being identified in younger patients, at a lower grade, and when there is organ-confined disease (see the “Prostate Cancer Screening” section).3 Due to the long natural history of prostate cancer, the average duration between biochemical recurrence following radical prostatectomy and death from prostate cancer is 13 years.4 This means that many patients who received a diagnosis of prostate cancer when they were younger will be elderly by the time they are being treated for disease relapse and progression.
Age and Undertreatment
Elderly patients with prostate cancer may not receive the optimum care if treatment decisions are based solely on chronological age. Alibhai et al5 studied age-specific treatment patterns for localized prostate cancer using the Ontario Cancer Registry and found that there was a decreased likelihood of receiving curative therapy correlating with increased age, Charlson Comorbidity Index score, and tumor stage. Despite similar remaining life expectancies, older men were less likely to receive curative treatment. Of those who received curative treatment and had a life expectancy of ≥10 additional years, approximately 73% were <60 years of age, approximately 68% were 60 to 69 years of age, and only 40% were 70 to 79 years of age.5
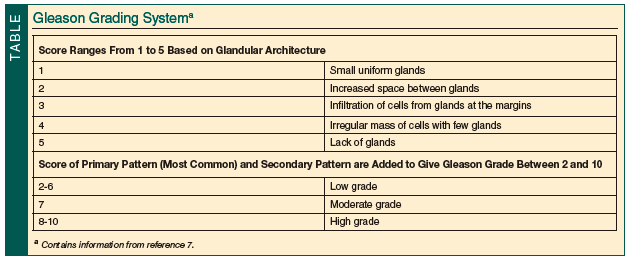
Schwartz et al6 reviewed the factors influencing treatment decisions for localized prostate cancer and found that age, comorbidity, and Gleason score (Table7) were independent predictors of suboptimal treatment. Men ≥70 years of age with a moderately or poorly differentiated tumor and no or mild comorbidity were treated subobtimally with watchful waiting when curative treatments were feasible. Dahm et al8 studied 484 patients age ≥70 years who were undergoing radical perineal prostatectomy for organ-confined prostate cancer. These men had a significantly lower risk of death from prostate cancer compared with patients undergoing watchful waiting. The subgroup analysis by Gleason score of patients with a life expectancy of ≥10 years showed a similar risk of death as younger patients. Finally, Thompson et al9 demonstrated that patients ≥80 years of age could achieve satisfactory functional and oncological outcomes after radical surgery for localized prostate cancer.
These data indicate that age should not be used as the sole criterion when making treatment decisions for patients with prostate cancer. Overall health status, including the presence of competing comorbid conditions, must also be considered.
Comorbidities
Comorbidity is a crucial predictor of life expectancy and non–prostate cancer mortality. Sweat et al10 demonstrated in their retrospective analysis that for any given Gleason score, a healthy elderly patient had a similar prostate cancer mortality as a healthy patient <60 years of age. Non–prostate cancer mortality was strongly related to the degree of comorbidity, independent of age. Tewari et al11 showed that the risk of non–prostate cancer mortality was three times greater in patients with severe comorbidities and prostate cancer compared with those with fewer comorbidities.
The limitations of chronological age and comorbidity indices cannot account for aging as a heterogeneous and individualized process. Extermann et al12 have reported on the concept of the comprehensive geriatric assessment (CGA) in the oncology setting. CGA is a “multidisciplinary evaluation in which the multiple problems of the older persons are uncovered, described, and explained if possible, and in which the resources and strengths of the person are catalogued, need for services assessed, and a coordinated care plan developed to focus interventions on the person’s problems.”13 CGA is a comprehensive process that includes procedures, treatment plans, and intervention. The domains that are typically assessed include cognition, comorbidity, emotional conditions, functional status, geriatric syndromes, medications, nutrition, and socioeconomic conditions. Randomized trials using CGA have demonstrated a benefit from CGA on survival, quality of life, and institutionalization.12
CGA permits the classification of patients with cancer into four groups: (1) patients who are fit and should receive the same treatment as younger patients; (2) patients who are vulnerable and should receive standard treatment after geriatric/medical intervention; (3) patients who are frail and should receive adapted treatments (mean treatments that the patient can tolerate but may not be first line or aggressive); and (4) patients who are too sick to receive standard treatment or adapted treatment and should receive symptomatic palliative treatments.14 The detailed evaluation of patients helps predict the probability of survival based on specific health status criteria. The CGA is in development and is being studied in the geriatric oncology setting, but the current evidence generally supports the benefits of this approach.
Age, comorbidities, and CGA or other health status evaluations should be used to individualize a patient’s assessment and treatment for prostate cancer.
Prostate Cancer Screening
PSA testing and digital rectal examination (DRE) can aid in the detection of prostate cancer before it becomes clinically evident. Screening for prostate cancer with PSA, however, remains a controversial topic.
Screening with PSA has led to a substantial shift toward the detection of prostate cancer at an earlier stage.15 Evidence from a randomized trial in Sweden16 and a well-controlled cohort study in the United States17 supports the contention that treatment of localized prostate cancer can reduce prostate cancer–specific mortality. These studies also support the early detection and subsequent treatment of localized prostate cancer.
The ERSPC (European Randomized Study of Screening for Prostate Cancer)18 demonstrated a 20% reduced mortality rate in the group that received PSA screening. The study estimated that 1410 men would need to be screened and another 48 men would need to be treated for the prevention of one prostate cancer death over a 10-year period. The PLCO (Prostate, Lung, Colorectal, and Ovarian) Cancer Screening Trial19 demonstrated no difference in prostate cancer death at 7 years of follow-up between screened and nonscreened groups.
Based on these studies, the American Urological Association (AUA) issued a best practice statement that advocates PSA screening20:
Given the uncertainty that PSA testing results in more benefit than harm, a thoughtful and broad approach to PSA is critical. Patients need to be informed of the risks and benefits of testing before it is undertaken. The risks of overdetection and overtreatment should be included in this discussion. Because there is now evidence from a randomized, controlled trial regarding a mortality decrease associated with PSA screening, the AUA is recommending PSA screening, as proposed in this document, for well-informed men who wish to pursue early diagnosis.
Vickers et al21 published a study that supported that PSA concentration at age 60 years predicts lifetime risk of metastasis and death from prostate cancer. The study showed that men who have a PSA concentration of ≤1 ng/mL may have prostate cancer, but it is very unlikely ever to become life-threatening. Only a minority of men with PSA concentrations in the top quarter (>2 ng/mL) developed fatal prostate cancer, and 90% of deaths from prostate cancer occurred in these men. These data imply that men with a PSA concentration of >2 ng/mL at age 60 years should be more aggressively screened, and those with a PSA concentration of ≤1 ng/mL may not require rigorous screening programs. The study does have limitations, such as higher screening age, low screening rates, overestimation of clinically detected cancer, and a homogenous cohort.
Prostate cancer incidence and mortality both increase with age, but so does non–prostate cancer mortality. Therefore, it stands to reason that the benefit from screening and treatment decreases with increasing age. The median time from diagnosis to death in persons with nonpalpable prostate cancer is 17 years.22 A Canadian study that examined men age ≥70 years and ≥80 years concluded that 40% of men selected for radical prostatectomy did not have adequate life expectancy to warrant treatment.23 Additionally, 70% of men who underwent radiation therapy died before reaching the 10-year survival mark. Contrary to these studies, Wong et al17 demonstrated that men age 65 to 80 years had a survival advantage with active treatment of low- and intermediate-risk prostate cancer compared with those in the observation group.
Clearly, the debate regarding prostate cancer screening is confusing. Furthermore, how it applies to the elderly population is difficult to analyze, as some studies testing the outcomes of screening do not include patients ≥75 years of age.24 The AUA recommends that screening begin at age 50 years for men at average risk for prostate cancer and at age 40 years for men who are African American or have a family history of prostate cancer in a first-degree relative.20 The American College of Physicians has deferred its recommendation on screening to the U.S. Preventive Services Task Force, which advises against screening for prostate cancer in men ≥75 years of age.25 Konety et al26 published a consensus statement for screening men who are >75 years of age and highlighted the following considerations when determining whether to initiate or continue screening: (1) risk factors for prostate cancer; (2) survival benefit from treatment; (3) whether the patient is likely to pursue therapy; (4) use of age-adjusted PSA values; and (5) a discussion that screening may not prolong survival.
Elderly men should be screened for prostate cancer selectively based on an overall health assessment. Patients should understand the goals and benefits of screening. Pitfalls of screening, such as additional testing, overdiagnosis, and overtreatment, should also be explained. Age- and race-adjusted reference PSA ranges should be used when screening to improve the sensitivity and possibly the specificity of PSA screening: Asians, 0 to 4.0 ng/mL for ages 60 to 69 years and 0 to 5.0 ng/mL for ages 70 to 79; African Americans, 0 to 4.5 ng/mL for ages 60 to 69 years and 0 to 5.5 ng/mL for ages 70 to 79 ng/mL; and whites, 0 to 4.5 ng/mL for ages 60 to 69 years and 0 to 6.5 ng/mL for ages 70 to 79.27
DRE is also an important aspect of prostate cancer screening. It involves transrectal palpation of the posterior aspect of the prostate gland by the examiner’s finger to feel for nodules, symmetry, consistency, and irregularity. Catalona et al28 published a large series examining the performance of PSA and DRE as screening tools for prostate cancer. The detection rate was 75% for PSA and 56% for DRE when used separately. In combination, the detection rate increased to 78%, supporting the combination of DRE and PSA for screening.
Staging, Grading, and Prognostic Considerations
Patients should have a transrectal ultrasound–guided biopsy of the prostate gland if the DRE is abnormal or elevated PSA levels are detected. Staging methods for the detection of metastasis are tailored to the patient’s disease. All patients should undergo routine blood analysis, including liver function tests. Body imaging with computed tomography or magnetic resonance imaging (MRI) and bone scans should rarely be used for patients with low-risk disease, but they are considered standard for patients with higher-risk disease. Endorectal MRI can also be used for the assessment of locally invasive disease, for the assessment of capsular invasion, or for targeting biopsies. The tumor, node, metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer is the most widely accepted and used criteria for staging prostate cancer.29
The treatment of prostate cancer requires a risk assessment of negative oncological outcomes. Clinical stage, PSA, and Gleason grade are the most widely used criteria for risk assessment. The D’Amico criteria30 stratifies patients into low-, moderate-, and high-risk groups. These criteria have been adopted by the AUA and the AJCC. The stratification is based on 5-year PSA failure rates. Patients in the low-risk group have a <25% chance of 5-year PSA failure (disease recurrence based on PSA), PSA level of ≤10 ng/mL, Gleason sum of ≤6, and clinical stage of T1c or T2a. Patients in the intermediate-risk group have a 25% to 50% chance of 5-year PSA failure, PSA level of 10 to 20 ng/mL, Gleason sum of 7, and clinical stage of T2b. Patients in the high-risk group have a >50% chance of 5-year PSA failure, PSA level of >20 ng/mL, Gleason sum of 8 to 10, and clinical stage of T2c or higher.
There are several other prognostic tools, such as PSA velocity (the rate of change in the PSA level), PSA doubling time (the length of time it takes for a PSA level to double), estimation of tumor volume, and number of positive cores on prostate biopsy. There are also models and nomograms in widespread use, which are easily accessible on the Internet (Partin tables, Memorial Sloan-Kettering Cancer Center [MSKCC] nomogram). These tools are helpful in predicting PSA failure, disease recurrence, probability of extraprostatic disease, nodal movement, and metastatic potential.
Treatment of Localized Prostate Cancer
Treatment strategies for localized prostate cancer include active surveillance, radical prostatectomy, external beam radiation therapy (EBRT), and brachytherapy. Other, less frequently used treatment strategies, such as cryotherapy and high intensity–focused ultrasound, are also available but are beyond the scope of this article.
Continued on next page
Active Surveillance
Active surveillance implies continued monitoring of prostate cancer without immediate treatment. Watchful waiting is a similar term. Historically, these terms have been used interchangeably. Active surveillance, however, is the favored term used for patients whose cancer is being actively monitored with the intent or option to treat on progression. Patients who choose active surveillance are making the judgment that immediate treatment will not benefit their oncological outcome or that potential treatment side effects are not worth the risk of treatment. Patients will undergo PSA testing and repeat biopsies at predetermined intervals after they receive a diagnosis of prostate cancer. If rising PSA values are observed, more frequent or earlier assessments are undertaken. Patients can continue with or change their treatment choice based on a continual reassessment of their disease and rationale for treatment.
Active surveillance is a valid treatment option for the elderly. The approach may be particularly applicable to patients with low- or even intermediate-risk disease. Albertsen et al31 studied patients with localized prostate cancer who were treated with immediate or delayed hormonal therapy and followed for 20 years. Patients did not undergo any other treatments. In patients 70 to 74 years of age, only 22% died of prostate cancer if they had a Gleason score of ≤6, 40% if they had a Gleason score of 7, and 64% if they had a Gleason score of 8 to 10.
Active surveillance studies show promising results, and cancer-specific survival ranges from 95% to 100% over follow-up periods of up to 10 years.32 Klotz et al33 studied 450 men with low-risk prostate cancer. The study also included men >70 years who had a Gleason sum of 3+4=7 or a PSA of ≤15 ng/mL and a clinical stage of T1b to T2bN0M0. The mean age of patients was 70 years. Patients were followed up with serial PSA screenings and repeat biopsies. Treatment was initiated in cases of a rapid PSA rise, observation of disease progression on prostate biopsy, or when requested by the patient. The 10-year rate of cancer-specific survival in this study was 97%.
Careful selection of patients who are at low risk for cancer progression or cancer-specific death is one of the major contributors to the success of active surveillance. The PRIAS (Prostate Cancer Research International: Active Surveillance) group34 has recommended the following inclusion criteria for active surveillance: PSA level of ≤10.0 ng/mL, a PSA density (PSA divided by prostate volume) of <0.2 ng/mL/mL, T1c or T2 disease, and one or two positive prostate needle biopsy cores, with a Gleason score of 3+3=6. PSA should be obtained every 3 months, and repeat biopsies should be performed at an interval of 12 to 24 months.32 Treatment is initiated if there is a rapid rise in PSA level or if progression is detected on repeat biopsy.32
There can be an element of psychological distress due to an untreated malignancy in patients undergoing active surveillance. Patient wishes—and not oncological criteria—account for the vast majority of the 20% to 30% of patients in whom treatment is initiated within 2 to 3 years of starting active surveillance.32 Thorough patient education is very important to adherence, which tends to be higher among elderly patients.35
Continued on next page
Radical Prostatectomy
Radical prostatectomy involves the complete removal of the prostate gland, the attached seminal vesicles, and the ampulla of the vas deferens. Under general anesthesia, the procedure can be performed through a retropubic, perineal, laparoscopic, or robotic approach. The major potential advantage of prostate removal in truly localized disease is cancer cure.
A 2008 randomized trial of 695 men with a mean age of 65 years compared radical prostatectomy to watchful waiting.36 Radical prostatectomy reduced prostate cancer mortality and the risk of developing metastases; however, researchers found no significant benefit in overall survival within the first 10 years after surgery in men ≥65 years of age. Consideration also has to be given to evidence that postoperative complications increase with age and degree of comorbidities.37
Urinary incontinence is a common side effect of radical prostatectomy. Increasing age is an established risk factor for long-term incontinence after radical prostatectomy.38 Development of urinary incontinence in older adults may lead to limitations in physical activity and social interaction, which may negatively influence both overall and health-related quality of life. In some cases, postoperative urinary incontinence can be effectively managed with pelvic floor muscle exercises and other behavioral techniques. Some men with severe urinary incontinence, however, may require additional surgical management.
Erectile dysfunction can also occur after radical prostatectomy. These changes in sexual function can have negative effects on quality of life in elderly men. The recovery of erectile function is multivariable, affected by patient age, preoperative erectile function, erectile hemodynamic changes after surgery, quality of surgical neurovascular bundle preservation, and surgeon volume.39
In elderly patients, radical prostatectomy is a reasonable option for those at high risk of prostate cancer death, provided they are good surgical candidates (“fit” or “vulnerable” according to CGA criteria) and have a low risk of developing incontinence.38 The AUA Guideline for the Management of Clinically Localized Prostate Cancer40 states that patients most likely to benefit from radical prostatectomy are those with a relatively long life expectancy, no significant surgical risk factors, and a preference to undergo surgery.
External Beam Radiation Therapy
EBRT is widely accepted as the therapy of choice for elderly men with intermediate- and high-risk prostate cancer.35 Several studies have reported similar functional, oncological, and treatment-related outcomes when comparing elderly patients with younger patients.38 In an analysis of 527 patients, researchers reported no age-related differences in the risk of acute or late genitourinary or gastrointestinal toxicity after EBRT.41
Age at diagnosis has been shown to be an independent predictor of prostate cancer death for patients undergoing EBRT for treatment of localized prostate cancer.42 D’Amico et al42 found that the median time to death from prostate cancer in high-risk patients who were ≥75 years of age at diagnosis was 6.3 years compared with 9.7 years for younger patients. Randomized trials have also demonstrated the superiority of combination treatment with EBRT and androgen deprivation therapy (ADT) for 2 to 3 years compared with monotherapy with EBRT in men with intermediate- and high-risk prostate cancer.43 It has been shown that the survival advantage of combination treatment for high-risk cancer may apply only to those patients with minimal or no comorbidities.44
The AUA guideline recommendations on EBRT are similar to the recommendations on radical prostatectomy. They advocate that men who are most likely to benefit from EBRT are those with a relatively long life expectancy, no significant risk factors for radiation toxicity (inflammatory bowel disease, Crohn’s disease, ulcerative colitis, previous pelvic radiotherapy), and a preference to undergo radiotherapy.40
Brachytherapy
Brachytherapy involves the surgical implantation of radioactive seeds into the prostate using a percutaneous needle approach inserted through the perineum. Brachytherapy is indicated for localized prostate cancer and is generally used in low-risk patients; however, its application has extended to intermediate-risk patients.45 Although elderly patients are well suited to undergo brachytherapy, evidence is lacking regarding its advantage on overall survival.
The safety and efficacy of brachytherapy have been established and encouraging long-term data have been provided in trials.45 Although treatment side effects of brachytherapy are less pronounced than side effects of EBRT or radical prostatectomy, they increase with both age and comorbidities.46 However, a patient’s score on the Charlson Comorbidity Index is a stronger predictor of complications than age.46 When used in its most-accepted low-risk setting, the criteria for brachytherapy are very similar to those of active surveillance; thus, its application in elderly patients is questionable.46
Treatment of Advanced Prostate Cancer
The mainstay of treatment for metastatic prostate cancer is ADT. Other options include chemotherapy and immunotherapy (the latter of which is beyond the scope of this article).
Hormonal Therapy
The known benefits of ADT are delayed progression, palliation of symptoms, and potential prevention of catastrophic complications such as spinal cord compression caused by osteoblastic bone lesions.38 First-line hormonal therapy, although widely used, is seldom indicated in patients with localized prostate cancer.40 Although many men are started on ADT at diagnosis of metastatic disease, there are no data to support this practice. ADT does not appear to offer a survival advantage.47 Overall survival is similar in men who receive immediate or deferred ADT.48 Although patients who receive immediate ADT have lower prostate cancer–related complications, potential side effects of long-term ADT such as osteoporosis, fractures, loss of libido, memory loss, depression, metabolic syndrome, and cardiovascular disease must be considered.48 Men receiving ADT are also more likely to have depressive, cognitive, and constitutional disorders; however, these conditions can often be attributed primarily to the fact that men receiving ADT are generally older and have more comorbidities.49
ADT can be accomplished by bilateral orchiectomy, the administration of luteinizing hormone–releasing hormone (LHRH) agonists, or the administration of LHRH antagonists. All clinical approaches are acceptable and there are no established differences in terms of cancer outcomes. The use of LHRH agonists is more widely preferred due to physical and psychological implications associated with orchiectomy.50 LHRH antagonists are safe and effective in achieving rapid castrate testosterone levels. They have the advantage of eliminating flare prophylaxis, but they often have to be given more frequently (monthly) than LHRH agonists (3-4 months).51
Patients receiving ADT should be placed on preventive strategies and treatment for osteoporosis. Annual bone mineral density evaluation with dual energy X-ray absorptiometry scan and treatment with calcium and vitamin D supplementation are recommended. Men with a higher risk of fractures should be considered for treatment with bisphosphonates.52
Chemotherapy
Elderly men with hormone-refractory prostate cancer should be treated in the same manner as comparable younger patients. Chemotherapy with docetaxel plus low-dose prednisone is the standard therapy, and it demonstrates the same efficacy in healthy elderly patients as it does in younger men.53 Neutropenia, fatigue, alopecia, and constitutional symptoms are common side effects of chemotherapy.53
Conclusion
Prostate cancer is a highly prevalent condition in older men, and rates increase with advancing age. Although many men diagnosed with prostate cancer will ultimately succumb to other comorbid conditions, proper evaluation and management of the disease are essential. Screening for prostate cancer, particularly among elderly men, remains controversial, although guidelines are being developed. Continued research will help clarify the role of screening in this population.
In elderly men with prostate cancer, the choice of definitive therapy is usually guided by a combination of factors, including cancer-specific characteristics, overall comorbidity, projected remaining life expectancy, and the patient’s treatment goals. Even in elderly men who are not candidates for definitive therapy, establishing the diagnosis is useful in helping to plan hormonal or other forms of therapy. This may have important consequences in terms of helping to foster improved overall and health-related quality of life.
Older adults represent the fastest-growing segment of the US population. The aging of the “Baby Boom” generation will lead to a significant increase in the number of older adults in our society. In turn, this will lead to increased utilization of healthcare resources. These shifting population demographics mean that more men will likely be diagnosed with prostate cancer in the future. Geriatricians and other healthcare providers treating the older patient should be aware of the options for the evaluation and management of this condition in elderly men and should tailor their approach to each individual patient.
The authors report no relevant financial relationships.
Dr. Mirza is Assistant Professor, Department of Urology, and Dr. Griebling is the John P. Wolf 33° Masonic Distinguished Professor and Vice Chair, Department of Urology, and Faculty Associate, Landon Center on Aging, The University of Kansas, Kansas City.
References
1. Altekruse SF, Kosary CL, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2007. National Cancer Institute. Bethesda, MA. http://seer.cancer.gov/csr/1975_2007/. Based on November 2009 SEER data submission. Posted to the SEER Website, 2010.
2. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2008. National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2008/. Based on November 2010 SEER data submission. Posted to the SEER Website, 2011.
3. Paquette EL, Sun L, Paquette LR, Connelly R, Mcleod DG, Moul JW. Improved prostate cancer-specific survival and other disease parameters: impact of prostate cancer-specific testing. Urology. 2002;60(5):756-759.
4. Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591-1597.
5. Alibhai SMH, Krahn MD, Cohen MM, Fleshner NE, Tomlinson GA, Naglie G. Is there age bias in the treatment of localized prostate carcinoma? Cancer. 2004;100(1):72-81.
6. Schwartz KL, Alibhai SMH, Tomlinson G, Naglie G, Krahn MD. Continued undertreatment of older men with localized prostate cancer. Urology. 2003;62(5):860-865.
7. Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50(3):125-128.
8. Dahm P, Silverstein AD, Weizer AZ, Crisci A, Vieweg J, Paulson DF. When to diagnose and how to treat prostate cancer in the “not too fit” elderly. Crit Rev Oncol Hematol. 2003;48(2):123-131.
9. Thompson RH, Slezak JM, Webster WS, Lieber MM. Radical prostatectomy for octogenarians: how old is too old? Urology. 2006;68(5):1042-1045.
10. Sweat SD, Bergstralh EJ, Slezak J, Blute ML, Zincke H. Competing risk analysis after radical prostatectomy for clinically nonmetastatic prostate adenocarcinoma according to clinical Gleason score and patient age. J Urol. 2002;168(2):525-529.
11. Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171(4):1513-1519.
12. Extermann M, Aapro M, Bernabei R, et al; Task Force on CGA of the International Society of Geriatric Oncology. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005;55(3):241-252.
13. National Institutes of Health Consensus Development Conference Statement: geriatric assessment methods for clinical decision-making. J Am Geriatr Soc. 1988;36(4):342-347.
14. Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5(3):224-237.
15. Etzioni R, Gulati R, Falcon S, Penson DF. Impact of PSA screening on the incidence of advanced stage prostate cancer in the United States: a surveillance modeling approach. Med Decis Making. 2008;28(3):323-331.
16. Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977-1984.
17. Wong Y, Mitra N, Hudes G, et al. Survival associated with treatment vs. observation of localized prostate cancer in elderly men [published correction appears in JAMA. 2007;297(1):42]. JAMA. 2006;296(22):2683-2693.
18. Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320-1328.
19. Andriole GL, Crawford ED, Grubb RL III, et al; PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial [published correction appears in N Engl J Med. 2009;360(17):1797]. N Engl J Med. 2009;360(13): 310-1319.
20. Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182(5):2232-2241.
21. Vickers AJ, Cronin AM, Björk T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ. 2010;341:c4521. doi:10.1136/bmj.c4521.
22. Horan AH, McGehee M. Mean time to cancer-specific death of apparently clinically localized prostate cancer: policy implications for threshold ages in prostate-specific antigen screening and ablative therapy. BJU Int. 2000;85(9):1063-1066.
23. Jeldres C, Suardi N, Walz J, et al. Poor overall survival in septa- and octogenarian patients after radical prostatectomy and radiotherapy for prostate cancer: a population-based study of 6183 men. Eur Urol. 2008;54(1):107-116.
24. Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomized controlled trials. BMJ. 2010;341:c4543.
25. U.S. Preventative Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(3):185-191.
26. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
27. Richardson TD, Oesterling JE. Age-specific reference ranges for serum prostate-specific antigen. Urol Clin North Am. 1997;24(2):339-351.
28. Catalona WJ, Richie JP, Ahmann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151(5):1283-1290.
29. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. Seventh edition. New York, NY: Springer; 2010.
30. D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol. 2003;21(11):2163-2172.
31. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095-2101.
32. Adolfsson J. Watchful waiting and active surveillance: the current position. BJU Int. 2008;102(1):10-14.
33. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126-131.
34. van den Bergh RC, Vasarainen H, van der Poel HG, et al. Short-term outcomes of the prospective multicentre ‘Prostate Cancer Research International: Active Surveillance’ study. BJU Int. 2010;105(7):956-962.
35. Berger I, Böhmer F, Ponholzer A, Madersbacher S. Prostate cancer in senior adults: over- or undertreated? Wien Med Wochenschr. 2009;159(21-22):521-528.
36. Bill-Axelson A, Holmberg L, Filen F, et al; Scandinavian Prostate Cancer Group Study Number 4. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144-1154.
37. Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138-1144.
38. Droz JP, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73(1):68-91.
39. Mulhall JP. Defining and reporting erectile function outcomes after radical prostatectomy: challenges and misconceptions. J Urol. 2009;181(2):462-471.
40. Thompson I, Thrasher JB, Aus G, et al; AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
41. Jani AB, Parikh SD, Vijaykumar S, Gratzle J. Analysis of influence of age on acute and chronic radiotherapy toxicity in treatment of prostate cancer. Urology. 2005;65(6):1157-1162.
42. D’Amico AV, Cote K, Loffredo M, Renshaw AA, Chen MH. Advanced age at diagnosis is an independent predictor of time to death from prostate carcinoma for patients undergoing external beam radiation therapy for clinically localized prostate carcinoma. Cancer. 2003;97(1):56-62.
43. Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103-106.
44. D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs. radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299(3):289-295.
45. Cosset JM, Flam T, Thiounn N, et al. Permanent implant prostate cancer brachytherapy [in French]. Cancer Radiother. 2008;12(6-7):503-511.
46. Chen AB, D’Amico AV, Neville BA, Earle CC. Patient and treatment factors associated with complications after prostate brachytherapy. J Clin Oncol. 2006;24(33):5298-5304.
47. Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355(9214):1491-1498.
48. The Medical Research Council Prostate Cancer Working Party Investigators Group. Immediate versus deferred treatment for advanced prostate cancer: initial results of the Medical Research Council Trial. Br J Urol. 1997;79(2):235-246.
49. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166(4):465-471.
50. Heidenreich A, Aus G, Bolla M, et al; European Association of Urology. EAU guidelines on prostate cancer. Eur Urol. 2008;53(1):68-80.
51. Crawford ED, Hou AH. The role of LHRH antagonists in the treatment of prostate cancer. Oncology (Wilson Park). 2009;23(7):626-630.
52. National Comprehensive Cancer Network. NCCN oncology guidelines 2010: prostate cancer. Available at: http://www.nccn.org. Accessed July 29, 2011.
53. Tannock IF, de Wit R, Berry WR, et al; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.


