Optimizing Post-Resuscitation Care in the Emergency Department: Addressing Post-Resuscitation Syndrome With a Structured Early Goal-Directed Approach
ABSTRACT: Worldwide survival rates from out-of-hospital cardiac arrest (OHCA) are low, despite great advances in resuscitation science research and technology. For reasons that are complex and multifactorial, many hospitals around the world do not provide systematic, structured post-resuscitation care, thereby contributing to the overall decreased survival rates. With the goal of neurologically intact survival, optimal in-hospital therapies should begin in the emergency department and center on interventions that are evidence-based and proven to maximize survival. This article, which is the first of a 2-part series, provides a brief overview of post-resuscitation syndrome (PRS), a major contributor to poor outcomes among patients who have had an OHCA. It also describes the goals of managing the pathophysiological processes associated with PRS when using a standardized systems-of-care approach that emphasizes early goal-directed therapies.
In regions worldwide, neurologically intact survival from out-of-hospital cardiac arrest (OHCA) is uncommon, despite recent strides in resuscitation science. Yet, when optimal out-of-hospital care is provided to patients with OHCA from sudden ventricular fibrillation (VF) or pulseless ventricular tachycardia (pVT), survival rates improve dramatically. Such care includes the early activation of the emergency response team, bystander/healthcare provider-initiated continuous chest compressions and cardiopulmonary resuscitation (CPR), and rapid defibrillation. Collectively, these interventions provide patients with OHCA the greatest chance for a return of spontaneous circulation (ROSC).
Optimal post-resuscitation care, whether in- or out-of-hospital, begins with the achievement of ROSC, with a final goal of long-term, neurologically intact survival 1 year after hospital discharge. Healthcare providers face a daunting task, as 75% of patients with OHCA who achieve ROSC in the field die before hospital discharge.1 Patients with OHCA are particularly vulnerable to poor outcomes following resuscitation due to post-resuscitation syndrome (PRS), which comprises a unique and complex combination of pathophysiological processes, including systemic ischemia/reperfusion response, neurologic injury, myocardial dysfunction, and the underlying cause of the cardiac arrest.
In industrialized countries, especially at tertiary care medical and research centers, optimal up-to-date, evidence-based care is frequently provided to these patients, but suboptimal care exists at many smaller hospitals and health clinics around the world. Numerous reasons have been cited for this, including political controversies at certain hospitals regarding the deployment of some post-resuscitation therapeutic options1,2 and variations in the care provided.3-8
It is clear that many hospitals worldwide are in need of a standardized, systematic, and structured approach to providing post-resuscitation care.1,2,9-16 This article will provide a brief overview of PRS, with an emphasis on the goals of care when implementing a standardized systems-of-care approach that promotes early goal-directed therapies (EGDTs).
Standardized Systems-of-Care Approach
An optimal systems-of-care approach to post-resuscitation care in the emergency department (ED) should include a well-organized, multidisciplinary strategy for all initially resuscitated OHCA patients—beginning in the out-of-hospital setting and continuing through the ED, the catheterization laboratory, and into the ICU. Many reports indicate that meeting appropriate post-ROSC patients at the door with aggressive, ED-based, bundled-care strategies increases favorable outcomes.1,3,4,10,11,17
A report by Sunde et al11 that compared a standard approach with an aggressive standardized post-resuscitation strategy found that 56% of those receiving the aggressive strategy had a favorable neurologic outcome and were still alive 1 year following hospital discharge compared with only 26% of those receiving the standard approach. In the study, the aggressive approach focused on maintaining and restoring vital organ function through EGDTs—such as therapeutic hypothermia, as-needed percutaneous coronary intervention (PCI), ventilation, seizure control, hemodynamic control, and maintenance of blood glucose levels. The individual components of PRS are potentially treatable and need to be addressed to improve outcomes.
In the setting of sepsis and septic shock, EGDTs are the standard of care and a proven method of organizing supportive care strategies.18 Due to the pathophysiologic similarities between sepsis and the patient’s state following resuscitation, an adapted EGDT approach has been used with great success in post-resuscitation care.19 Early induction of mild therapeutic hypothermia (MTH) has been long proven to significantly improve neurologic outcomes in survivors of OHCA.20 Likewise, early coronary angiography with PCI has been the mainstay of definitive care for post-ROSC patients with acute coronary syndrome (ACS), with a robust record for improving long-term survival outcomes.1,9-12,17,21-23 The International Liaison Committee on Resuscitation (ILCOR) and the American Heart Association (AHA) recommend that the routine care for initial survivors of OHCA should include the combination of aggressive supportive care strategies, MTH, and PCI.13,17,24 We propose an ED-based, standardized care approach for treating the post-resuscitation OHCA patient.
Overview of Post-Resuscitation Syndrome
As previously noted, PRS is a unique constellation of systemic pathophysiologic consequences, beginning during cardiac arrest and continuing into the post-ROSC period. First described in 1975 by Negovsky,6,25 it was termed post-cardiac arrest shock and attributed to severe systolic dysfunction. Today’s research has updated Negovsky’s original idea, and PRS is now considered a form of mixed shock, with both cardiogenic and vasodilatory properties and a presentation similar to septic shock.
Four key organ-specific components are associated with PRS and require attention: systemic ischemia and the reperfusion response, neurologic injury, myocardial dysfunction, and the arrest-precipitating pathology still persisting in the immediate post-resuscitation period (eg, ACS, pulmonary embolism, sepsis).6 In the past decade, resuscitation investigators have also vastly improved their understanding of PRS, gaining insight on the treatment modalities that are currently the most effective.6,26
Here is an overview of the effects of PRS on specific body systems. An understanding of these effects facilitates an understanding of the optimal therapeutic needs during the post-resuscitation period.
Systemic Ischemia/Reperfusion Response
During a cardiac arrest, a “no-flow” environment is produced, thus enabling severe ischemia to develop. With biosynthetic pathways eliminated and adenosine triphosphate generation stopped, membrane and pump dysfunction lead to cellular destruction via the release of calcium from failed voltage-gated channels, generation of free fatty acid, lactic acidosis, and oxidative stress.25 When the “low flow” situation is created, as during ROSC, the toxic metabolites are then circulated, and the increased oxygenation spawns cellular toxicity through large-scale induction of free radicals, cytokines, and proteases. In this setting, lipid peroxidation, apoptosis, and endothelial dysfunction ensues, depositing activated neutrophils in organ tissues and leading to multiorgan system failure.26
In addition, this endothelial dysfunction is associated with 2 other major consequences that further the failure.25
1. Systemic activation of the coagulation cascade, leading to microvascular thrombosis in all organ systems.
2. Potential GIflora invasion of failed microvascular regions of the splanchnic circulatory system, allowing GI
bacteria and endotoxins to disseminate systemically.
Direct endotoxin effect, coupled with increased capillary permeability from coagulation abnormalities, causes a sepsis-like condition with profound vasodilation, intravascular volume depletion, and increased susceptibility to infection.6
 Neurologic Injury
Neurologic Injury
The pathophysiologic changes discussed in the preceding paragraph play a direct role in the resulting neurologic injury that occurs after CPR. Additionally, ischemia resulting from the “no-flow” period, and hyperoxia leading to free radical damage during the reperfusion period, can collectively cause irreversible neuronal damage. These processes create a downward cycle. After neuron injury, increases in cerebral metabolic activity occur, which increases heat (pyrexia)—further injuring cells and perpetuating the cycle.27 Cerebral edema and cerebral microvascular collapse often occur, leading to increased cerebral vascular resistance, intravascular thrombus generation, and areas of infarction. Secondary cerebral injury also complicates CNS recovery because of hypoglycemia, seizures, and decreased cerebral perfusion pressures resulting from suboptimal hemodynamics.6
Myocardial Dysfunction
CPR is associated with significant systolic and diastolic dysfunction, a leading cause of post-ROSC mortality.6,28 The “no-flow” and “low-flow” states contribute to loss of myocardial perfusion and some degree of cellular injury. Even after normal myocardial blood flow is regained, an initial myocardial stunning effect—ie, reversible reduction of heart contraction following reperfusion that is not accounted for by tissue damage or reduced blood flow—is seen quickly after ROSC, manifesting as wall motion abnormalities causing increased central venous and pulmonary arterial pressures, and a decreased left ventricular ejection fraction.28
Nevertheless, multiple investigators have found that this stunning period is often completely reversible at 48 to 72 hours if normal myocardial blood flow is achieved early and aggressive supportive care is provided.6,25,28 However, if myocardial dysfunction is attributed to ACS, the wall motion abnormalities will persist or worsen if reperfusion strategies are not used.
OHCA-Precipitating Pathology
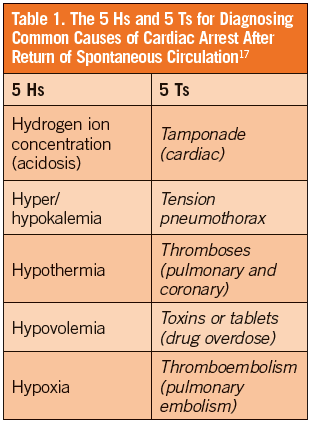
Survivors of cardiac arrest often present to the ED with a synergistic process that includes the PRS and the unresolved pathology that initially caused the arrest. Early, aggressive, supportive care can address the clinical features of PRS, but prompt diagnosis and management of the underlying pathology is imperative. Up to 71% of post-ROSC patients have coronary artery disease and almost 50% have an occluded coronary artery, making ACS the most common cause of sudden OHCA.1,5,6,21,27 Other common causes of pathology seen in post-ROSC patients can be assessed using the 5 Hs and 5 Ts mnemonic (Table 1).17
If diagnosed early, a theoretically simple solution may exist (eg, PCI for the patient with ACS or certain antidotes for the patient with drug overdose). Early treatment should be provided, preferably during the application of aggressive post-resuscitation care, and combined with neuroprotective strategies.
Post-Resuscitation Care and Treatment Goals
Optimal therapeutic goals should initially be achieved in the ED and include the provision of evidence-based care combined with the rapid mobilization of available resources. From the stable awake survivor to the unstable comatose patient with ST-elevation myocardial infarction, the treatment plan should be executed timely, with the priority being reversing the PRS pathology. While the definitive goal for most patients will be early coronary angiography and PCI coupled with MTH, all patients must be reasonably stabilized before PCI.
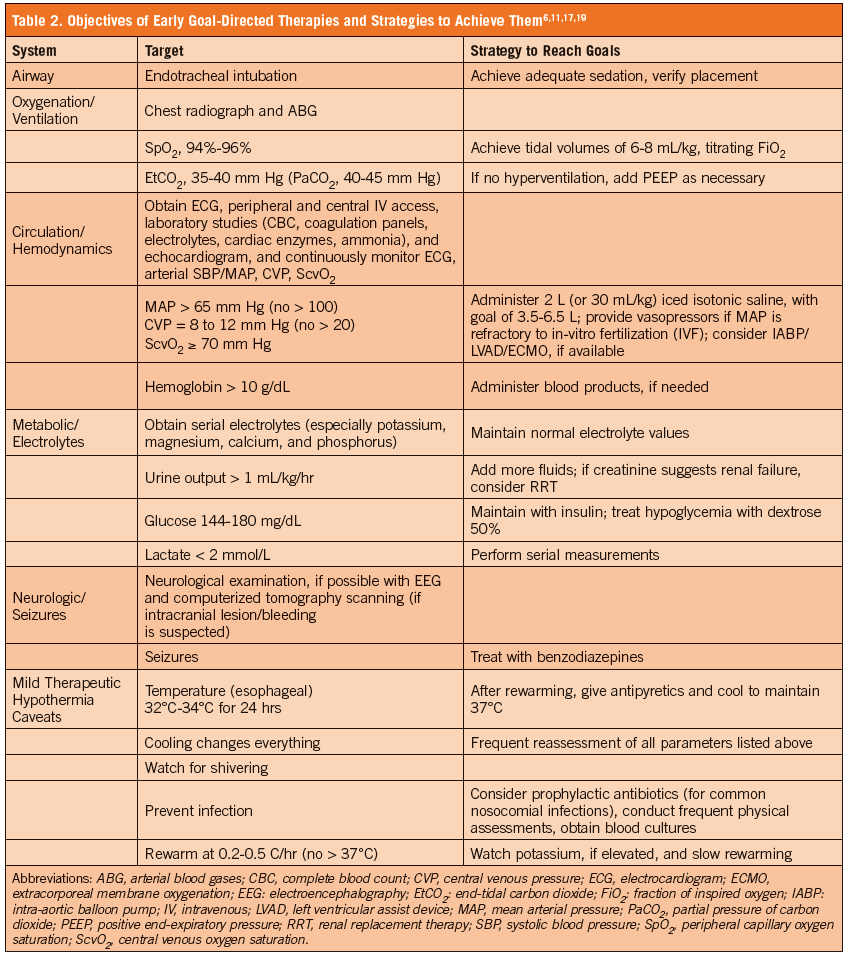
EGDT provides supportive care to the critically ill patient using end goals to optimize hemodynamics in a short period of time.19 This strategy reduces mortality in the setting of sepsis,18 and a similar approach has been recommended for the post-ROSC patient.6,11,17,19 Considering the overlap in pathophysiology between sepsis and PRS, a similar therapeutic approach seems reasonable, with specific goals outlined to optimize hemodynamics.29 Therefore, an early and aggressive focus should be centered on GDT, including airway stabilization, oxygenation/ventilation, hemodynam ics/circulation, electrolytes/metabolic measures, and neurologic status, while initiating MTH (Table 2).6,11,17,19
Airway
Aggressive airway management is of utmost importance to the ED physician. While the AHA and ILCOR have recently deemphasized aggressive airway management during the initial phases of a VF/pVT arrest, most post-ROSC OHCA patients will need an advanced airway secured upon arrival at the ED. For those patients with an airway placed by emergency medical services, endotracheal tubes should be confirmed by chest radiography and ongoing qualitative end-tidal carbon dioxide waveform monitoring; any supraglottic airway devices will eventually need to be replaced with a definitive airway.17
Oxygenation/Ventilation
Patients in the post-resuscitation period commonly present with pulmonary complications, including acute lung injury or acute respiratory distress syndrome.17 Diagnostic tests to consider include a chest radiograph and an arterial blood gas test. In general, ventilator settings should be based on oxyhemoglobin saturation, end-tidal carbon dioxide waveform monitoring, arterial blood gas values, and minute ventilation.
Patients frequently receive 100% oxygen during intra-arrest management to reverse and prevent myocardial and cerebral hypoxemia, but once an airway is established and oxygenation stabilized, the fraction of inspired oxygen during mechanical ventilation should be titrated to oxyhemoglobin saturation values between 94% and 96%. Multiple investigators suggest that hyperoxia maintained in the 100% range (particularly PaO2 ≥300 mm Hg) creates reactive oxygen species that may worsen neurologic injuries and functional outcomes.6,17,25,30 Minute ventilation rates should be carefully titrated to achieve tidal volumes of 6 mL/kg to 8 mL/kg and end-tidal carbon dioxide values of 35 mm Hg to 40 mm Hg (PaCO2, 40-45 mm Hg).17
Hyperventilation is a topic of debate and has 2 major disadvantages in the post-ROSC setting. First, fast ventilatory rates increase intrathoracic pressure, decreasing preload and cardiac output.31 Second, hypocapnia has been shown to decrease cerebral blood flow through excessive cerebral vasoconstriction, which can worsen cerebral ischemia.6,17 The only caveat is the patient with metabolic acidosis and a critically low pH value who, prior to intubation, is intrinsically compensating with hyperventilation. This patient may require hyperventilation after intubation to prevent dysrhythmias. However, in the majority of cases, routine hyperventilation is not recommended17 and plays no role in post-ROSC patient care.
Hemodynamics/Circulation
Patients frequently present with hemodynamic instability during the post-resuscitation period. While shock related only to myocardial stunning commonly improves or resolves completely after 48 hours to 72 hours, aggressive hemodynamic management is usually necessary to prevent organ ischemia and maintain coronary and cerebral perfusion pressures. Additionally, the precipitating cause of arrest should be quickly elucidated to optimize treatment.
If ACS is the presumed cause of myocardial dysfunction, no improvement will occur without PCI. Once airway, oxygenation, and ventilation are established, peripheral and central venous access should follow for intravascular crystalloid replacement, pharmacotherapy, and laboratory blood analysis (eg, complete blood count, electrolytes, serum lactate, cardiac enzymes). Cardiac monitoring should be continuous to detect recurrent dysrhythmias and a 12-lead EKG should be performed as early as possible.24
Invasive hemodynamic monitoring will be necessary to accurately assess central venous pressures, central venous oxygen saturation (ScvO2), and systolic/mean arterial pressure through an arterial line. To evaluate efficacy of tissue oxygenation and organ perfusion, urine output and ScvO2 should be monitored continuously and venous lactate levels should be drawn more than once to demonstrate a downward trend.
In 2010, sepsis investigators who studied continuous monitoring of ScvO2 versus lactate clearance as a measure of tissue oxygenation in patients with septic shock found no in-hospital mortality difference when tissue oxygenation was assessed by either method after initiation of resuscitation.32 Although this study was performed in the setting of septic shock, given the results, it may be reasonable for hospitals that do not have continuous ScvO2 monitoring capabilities to measure venous lactate clearance instead. As a caveat, seizure activity, hypothermia, and hepatic insufficiency may decrease lactate clearance; thus, continuous ScvO2 monitoring in the post-ROSC setting is recommended if at all possible.6 For the need to detect ventricular wall motion abnormalities or structural defects, echocardiography in the first 24 hours should be considered and might be useful in tailoring specific needs for pharmacologic or mechanical circulatory support.6,17,25
Shock in the post-ROSC patient should be aggressively managed, and can be a result of absolute or relative hypovolemia, myocardial dysfunction and vasodilation, or refractory dysrhythmias. Crystalloid replacement for hypovolemia in the first 24 hours can be as high as 3.5 L to 6.5 L, with the goal of normalizing systolic/mean arterial pressure (≥65 mm Hg), central venous pressure (8-12 mm Hg), and ScvO2 (≥70 mm Hg) or lactate clearance.16,33 If these goals cannot be met with fluids alone or if cardiogenic shock with pulmonary edema is present, vasoactive agents may be beneficial.6,17
For ScvO2 unresponsive to intravenous fluids, blood products may be necessary. When considering the complex pharmacokinetics of vasopressors, each specific drug has different chronotropic, inotropic, vasoconstrictive, and vasodilatory properties. While all vasoactive medications can increase myocardial oxygen demand in a dose-dependent manner, strong inotropic and chronotropic medications are more likely to have this risk.34 For shock refractory to pharmacologic management, intra-arterial balloon pump or percutaneous left-ventricular assist devices can be considered, if available; however, routine intra-arterial balloon pump deployment in the ED has not shown any significant increase in positive neurologic outcomes in the post-ROSC patient with refractory hypotension.17
Extracorporeal membrane oxygenation (ECMO) is an intriguing therapy that has shown some decrease in mortality in small case studies and retrospective reviews.35,36 The indications for use have historically been as in-hospital adjuvant therapy to CPR during prolonged cardiac arrest, so-called extracorporeal life support,37 but new interest is emerging regarding use of ECMO in profound shock states after ROSC. This may be an interesting therapy to monitor in the future as technology improves, but for now the availability is limited mostly to EDs in some tertiary centers, and few data are available regarding the routine use of ECMO in the ED for post-ROSC patients with shock.17,36
Finally, when shock is exacerbated by refractory dysrhythmias, the cause is likely related to myocardial ischemia in the setting of ACS. Antidysrhythmic and vasoactive medications can be used; the latter should be used carefully to avoid worsening ischemia or dysrhythmia, but most often the definitive antidysrhythmic therapy is PCI.6
Electrolytes/Metabolic Measures
EGDT is used to methodically correct hemodynamics, as discussed previously, and to address the critical electrolyte and metabolic disturbances that can occur during the post-resuscitation period. Many electrolytes will need to be monitored serially and replaced due to the dynamic nature of the post-ROSC period. Potassium, magnesium, calcium, and phosphate homeostasis should be reached, as abnormalities in these electrolytes can induce dysrhythmias. Serial lactate measurements can help to determine adequate tissue perfusion.6 Measurement of serum creatinine and urine output can assess whether any acute kidney injury is present, and whether renal replacement therapy is indicated. Maintaining diuresis at levels >1 mL/kg per hour will allow for assessment of euvolemia. Hyperammonemia has been associated with worsened neurologic outcomes, with 1 study finding levels >96 mg/dL to be an independent predictor of poor neurologic recovery in patients treated with MTH.38 Measurement of serum ammonia levels may be prudent for prognostic purposes, but no data are available regarding any potential efficacy in treating hyperammonemia.
Hyperglycemia is also common after cardiac arrest and left untreated, increases mortality and worsens neurologic outcomes.38 Therefore, blood glucose levels must be checked frequently, with the upper limit of normal maintained at or around 144 mg/dL to 180 mg/dL.6 Levels any higher require insulin therapy, with measures taken to prevent hypoglycemia with IV dextrose. Temperature monitoring for pyrexia in all post-ROSC patients should be a high priority, as sustained body temperatures ≥37.6°C are associated with poor survival.17 The obvious treatment goal for the comatose patient is early initiation of MTH. If pyrexia occurs after MTH (or in those who did not receive MTH), antipyretics should be given and controlled normothermia be initiated to maintain a body temperature of 37°C.27
Neurologic Status/Seizures
Most post-ROSC patients will be comatose upon arrival to the ED. Adequate sedation must be achieved to blunt the catecholamine response and the secondary effects yielded by the patient-ventilator interaction.17 If at all possible, and especially prior to neuromuscular blockade and deep sedation, a neurological examination should be attempted to determine baseline mental status and evaluate for seizures.
A cranial CT scan should be ordered immediately for any patient with a suspected intracranial pathology, but there are insufficient outcome data to suggest standardizing this intervention.26 Seizures and myoclonus are both common following ROSC,6 and both are common causes of pyrexia and further neurological injury in this setting. Although standardized anticonvulsant therapy is not recommended for seizure prevention, if seizures occur, consideration can be made for management with standard status epilepticus regimens.17 Additionally, any seizure should be evaluated by electroencephalography.6 All comatose patients with suspected seizure activity, especially those treated with neuromuscular blockers, should be evaluated by continuous electroencephalography monitoring.
PRS is a complex combination of pathophysiological processes that occur after CPR for cardiac arrest. The syndrome can lead to poor outcomes if any of its components are left unaddressed; therefore, every aspect of the syndrome must be carefully evaluated and treated using a structured, EGDT approach that starts in the field, continues into the ED, and is maintained through hospital discharge. In the conclusion of this 2-part series, the key treatments and considerations to optimize outcomes when managing PRS will be reviewed.
In Memoriam: This article is dedicated to Dr. Jake Benner, a selfless healthcare provider who died at a very early age. As a paramedic, he came to the aid of many, often serving as the first provider at the patient's side. During his short medical career, he accomplished more than most do over many decades. His impact was, and remains, considerable, as witnessed by this review of cardiac arrest management. He is most certainly missed, but most definitely not forgotten.
John P. Benner, DO, NREMT-P, was a graduate of the Edward Via College of Osteopathic Medicine in Blacksburg, VA.
James E. Powers, DO, is associate dean for clinical academic affairs, associate professor of emergency medicine, and chair of emergency medicine at the Edward Via College of Osteopathic Medicine in Blacksburg, VA.
David R. Burt, MD, is associate professor of emergency medicine at the University of Virginia and medical director of the Chest Pain Observation Unit at the University Medical Center, both in Charlottesville, VA.
William J. Brady, MD, is a professor of emergency medicine at the University of Virginia, as well as chair of the University Medical Center Resuscitation Committee and operational medical director with Albemarle County Fire Rescue, both in Charlottesville, VA.
References:
1.Kern KB. Optimal treatment of patients surviving out-of-hospital cardiac arrest. JACC Cardiovasc Interv. 2012;5(4):597-605.
2.Kern KB, Rahman O. Emergent percutaneous coronary intervention for resuscitated victims of out-of-hospital cardiac arrest. Catheter Cardiovasc Interv. 2010;75(4):616-624.
3.Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out-of-Hospital Cardiac ArresT) Registry. Circ Cardiovasc Interv. 2010;3(3):200-207.
4. Schefold JC, Storm C, Joerrs A, Hasper D. Mild therapeutic hypothermia after cardiac arrest and the risk of bleeding in patients with acute myocardial infarction. Int J Cardiol. 2009;132(3):387-391.
5. Kern KB. Establishing coronary patency: a key to optimal post resuscitation care. Signa Vitae. 2010;5(suppl 1):55-59.
6. Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A scientific statement from the international liaison committee on resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79(3):350-379.
7. Wang HE, Devlin SM, Sears GK, et al. Regional variations in early and late survival after out-of-hospital cardiac arrest. Resuscitation. 2012;83(11):1343-1348.
8. Carr BG, Kahn JM, Merchant RM, et al. Inter-hospital variability in post-cardiac arrest mortality. Resuscitation. 2009;80(1):30-34.
9. Cronier P, Vignon P, Bouferrache K, et al. Impact of routine percutaneous coronary intervention after out-of-hospital cardiac arrest due to ventricular fibrillation. Critical Care. 2011;15(3):R122.
10. Tømte Ø, Andersen GØ, Jacobsen D, et al. Strong and weak aspects of an established post-resuscitation protocol–a five-year observational study. Resuscitation. 2011;82(9):1186-1193.
11. Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardised treatment protocol for post-resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29-39.
12. Reynolds JC, Callaway CW, El Khoudary SR, et al. Coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. J Intensive Care Med. 2009;24(3):179-186.
13. O’Connor RE, Bossaert L, Arntz HR, et al. Part 9: acute coronary syndromes: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2010;122(suppl 2):S422-S465.
14. Banglore S, Hochman JS. A routine invasive strategy for out-of-hospital cardiac arrest survivors: are we there yet? Circ Cardiovasc Interv. 2010;3(3):197-199.
15. Merchant RM, Abella BS, Kahn M, et al. Cardiac catheterization is underutilized after in-hospital cardiac arrest. Resuscitation. 2008;79(3):398-403.
16. Nichol G, Aufderheide TP, Eigel B, et al. Regional systems of care for out-of-hospital cardiac arrest: a policy statement from the American Heart Association. Circulation. 2010;121(5):709-729.
17. Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(suppl 3):S768-S786.
18. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
19. Gaieski DF, Band RA, Abella BA, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418-424.
20. The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549-556.
21. Spaulding CM, Joly LM, Rosenberg A,et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336(23):1629-1633.
22. Dumas F, White L, Stubbs BA, et al. Long-term prognosis following resuscitation from out-of-hospital cardiac arrest: role of percutaneous coronary intervention and therapeutic hypothermia. J Am Coll Cardiol. 2012;60(1);21-27.
23. Batista LM, Lima FO, Januzzi Jr JL, et al. Feasibility and safety of combined percutaneous coronary intervention and therapeutic hypothermia following cardiac arrest. Resuscitation. 2010:81(4):398-403.
24. O’Connor RE, Brady WJ, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(suppl 3):S787-S817.
25. Mongardon N, Dumas F, Ricome S, et al. Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Ann Intensive Care. 2011;1(1):45.
26. Naples R, Ellison E, Brady WJ. Cranial computed tomography in the resuscitated patient with cardiac arrest. Am J Emerg Med. 2009;27(1):63-67.
27. Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(suppl 7):S186-S202.
28. Kern KB, Hillwig RA, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28(1):232-240.
29. Gaieski DF, Neumar RW, Fuchs B, et al. Haemodynamic management strategies are not explicitly defined in the majority of therapeutic hypothermia implementation studies. Resuscitation. 2012;83(7):835-839.
30. Neumar RW. Optimal oxygenation during and after cardiopulmonary resuscitation. Curr Opin Crit Care. 2011;17(3):236-240.
31. Aufderheide TP, Sigurdsson G, Pirrallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109(16):1960-1965.
32. Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs. central venous oxygen saturation as goals of early sepsis therapy. JAMA. 2010;303(8):739-746.
33. Wolfram S, Pierau C, Radke PW, et al. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med. 2008;36(6):1780-1786.
34. Overgaard CB, Dzavik V. Inotropes and vasopressors: review of physiology and clinical use in cardiovasculard. Circulation. 2008;118(10):1047-1056.
35. Chen YS, Chao A, Yu HY, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41(2):197-203.
36. Shinar Z, Bellezzo J, Paradis N, et al. Emergency department initiation of cardiopulmonary bypass: A case report and review of the literature. J Emerg Med. 2012;43(1):83-86.
37. Haneya A, Philipp A, Diez C, et al. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation. 2012;83(11):1331-1337.
38. Cho YM, Yong SL, Hyuk JY, et al. Blood ammonia is a predictive biomarker of neurologic outcome in cardiac arrest patients treated with therapeutic hypothermia. Am J Emerg Med. 2012;30(8):1395-1401.


