Peer Reviewed
Managing Dyslipidemia in Patients with Chronic Kidney Disease: Evidence-Based Guidance for Primary Care
When considering chronic kidney disease (CKD)—commonly defined as a sustained reduction in kidney function or evidence of kidney damage present for 3 months or longer—we often focus on the devastating effects associated with the late stages of the disease.1 Yet CKD is more than just end-stage renal disease (ESRD), and it often results in significant morbidity and mortality throughout the course of the disease.2 As CKD progresses, patients may present with, or be at risk for, comorbidities that include hypertension, cardiovascular disease (CVD), dyslipidemia, anemia, osteopenia and osteoporosis, nerve damage, and poor nutritional health.1
Managing these disorders should be an important focus of the primary care clinician treating patients with CKD. In particular, careful attention toward the meaningful increase in cardiovascular (CV) risk is warranted, including the clinical consequences of dyslipidemia. To optimize care, clinicians must understand the disparate pathophysiologic underpinnings of CV risk in CKD populations. In addition to recommended therapeutic lifestyle changes (TLC), clinicians must also be able to apply the primary outcomes of clinical lipid modulation trials to ensure the safe and effective management of dyslipidemia with statins, combined therapies, and adjunctive agents. After designing an individualized medication management strategy, clinicians need to consider approaches to enhance patient adherence to dyslipidemia therapy. Attention to these key aspects of care will enable clinicians to improve outcomes for their patients with CKD in the primary care setting.
DEFINING AND STAGING CKD
According to the Kidney Disease Outcomes Quality Initiative (K/DOQI) Task Force, CKD is manifested by markers of kidney damage, including defects in the composition of blood, urine, or imaging tests; or a glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 sustained for longer than 3 months.1 CKD is characterized by 5 stages of diminishing kidney function as measured by GFR (Table 1), beginning with stage 1 (normal kidney function) and ending in stage 5 (established kidney failure or ESRD).3 CKD is typically a progressive, lifelong condition that affects 26 million Americans, with millions more at risk of developing this condition.2 Primary care clinicians can minimize the associated morbidity and mortality by properly managing both CKD and its related comorbidities.2

THE CKD-CVD CONNECTION
Research has indicated that CVD risk increases with progressive decrements in renal function.4 Although it might seem intuitive that, as the GFR decreases, the risk of CV morbidity and mortality increases, the magnitude of CVD burden in this patient population is often underappreciated. A study by Go et al calculated GFR in 1,120,295 Kaiser Permanente Northern California members who had stage 1-4 CKD and found an independent, graded association between reduced GFR and the risk of death, CV events, and hospitalization.4 At median follow-up of 2.84 years, the risk of mortality in this patient population increased inversely with GFR: the adjusted hazard ratio for death was 1.2 with an estimated GFR of 45-59 mL/min/1.73 m2, 1.8 with an estimated GFR of 30-44 mL/min/1.73 m2, 3.2 with an estimated GFR of 15-29 mL/min/1.73 m2, and 5.9 with an estimated GFR of less than 15 mL/min/1.73 m2.4 In fact, results of this study determined that, compared with individuals with normal renal function (ie, GFR >60 mL/min/1.73 m2), patients with a GFR less than 15 mL/min/1.7 3 m2 are nearly 6 times more likely to have a CVD-related death, more than 3 times as likely to have a CV event, and 3 times as likely to be hospitalized.4
The importance of the CKD-CVD connection was recognized in a scientific statement published by the American Heart Association in 2003, which asserted that CKD is an independent risk factor for CVD development and that, “individuals with CKD are more likely to die of CVD than to develop kidney failure.”5 The authors note that the etiology of CVD risk in patients with CKD involves multiple, often overlapping risk factors such as diabetes, high blood pressure, anemia, and inflammation and the extended duration and severity of these conditions in this patient population.5 These risk factors are confounded in that patients with CKD often have undiagnosed vascular disease and are less likely to receive therapies proven to reduce CV risk, such as aspirin, beta blockers, angiotensin II converting enzyme inhibitors, and interventional procedures.5 Furthermore, the authors comment that traditional and nontraditional CVD risk factors increase in prevalence as kidney function declines; these include albuminuria, hyperhomocysteinemia, lipoprotein abnormalities, anemia and volume overload, abnormal calcium/phosphate metabolism, electrolyte imbalances, oxidant stress and inflammation, sleep disturbances, malnutrition, and thrombogenic factors.5
OVERVIEW OF CLINICAL LIPID MODULATION TRIALS IN PATIENTS WITH CKD
While the epidemiologic connection between CKD and CVD is strongly supported, relatively few studies have investigated interventions to prevent CVD specifically in CKD patients.6 Most subjects in large clinical trials evaluating the effect of lipid-lowering therapy on vascular events had either normal or only mildly reduced kidney function and an atherosclerotic cause of elevated CV risk.6 However, patients with CKD exhibit a unique CVD pathology, characterized by vascular stiffness and calcification, structural heart disease, and sympathetic nervous system overactivity. Whereas CV risk is certainly increased in the CKD population, the fact that the pathophysiology of CV events in this patient group is not always related to atherothrombotic plaque rupture raises the question as to whether lipid-lowering therapy provides sustained meaningful reduction in CV risk in CKD patients.
4D trial. In the Deutsche Diabetes Dialyse Studie (4D) trial, Wanner et al sought to evaluate the benefit of statin therapy in patients with ESRD.7 Patients with type 2 diabetes, a low-density lipoprotein cholesterol (LDL-C) level of 130 mg/dL or greater, and receiving hemodialysis were randomized in this multicenter, double-blind, prospective study to receive either atorvastatin (20 mg) daily or placebo (n51255).7 Although the median level of LDL-C was reduced by 42% in the atorvastatin group compared with 1.3% in the placebo group after 4 weeks of treatment, during a 4-year follow-up period, atorvastatin did not have a statistically significant effect on major adverse CV events (ie, CV death, fatal and nonfatal myocardial infarction [MI], and stroke) compared with placebo.7 Of the 469 patients who experienced such an event, 226 were in the atorvastatin cohort and 243 were in the placebo group (relative risk, 0.92; P5.37).7 Atorvastatin reduced the rate of cardiac events compared with placebo (relative risk, 0.82; P5.03), but did not have a significant effect on cerebrovascular events (relative risk, 1.12; P5.49) or total mortality (relative risk, 0.93; P5.33).7 Wanner et al concluded that, despite a trend toward CV risk reduction, atorvastatin did not have a statistically significant effect on CV death, nonfatal MI, and stroke in patients with diabetes receiving hemodialysis.7
AURORA. The AURORA trial (A study to evaluate the Use of Rosuvastatin in subjects On Regular hemodialysis: an Assessment of survival and cardiovascular events) also attempted to evaluate the effects of statin therapy in patients with ESRD.8 AURORA was an international, multicenter, randomized, double-blind, prospective trial involving 2776 patients undergoing hemodialysis.8 Three months after randomizing patients to receive either rosuvastatin (10 mg) daily or placebo, LDL-C was reduced by 43% in the active-treatment group compared with 2% among placebo recipients (P<.001).8 Study results after 3.8 years were similar to those reported in the 4D trial, with 396 patients in the rosuvastatin group and 408 patients in the placebo group suffering CV death, fatal and nonfatal MI, or stroke.7,8 As a result of the nonsignificant relative risk reduction (P5.59), the researchers determined that the initiation of treatment with rosuvastatin lowered LDL-C but had no significant effect on the composite primary end point of death from CV causes, nonfatal MI, or nonfatal stroke in patients undergoing hemodialysis.8
SHARP. The most recent major study to evaluate the potential effect of lipid-lowering therapies on CVD risk in individuals with CKD was the Study of Heart and Renal Protection (SHARP), a randomized, double-blind, placebo-controlled trial that investigated the effects of statins and statin combination therapy in individuals with moderate-to-severe CKD.6 Unlike the AURORA and 4D studies that were limited to the ESRD population, SHARP looked at a much wider population of patients with CKD; inclusion criteria for SHARP included a serum creatinine of at least 1.7 mg/dL for men and 1.5 mg/dL for women on at least 2 occasions.6 Additional inclusion criteria required patients to be at least 40 years of age with no known history of MI or coronary revascularization, and no definitive indication or contraindication to statin therapy.6 Baigent et al randomized 9270 patients with CKD (3023 of whom were receiving dialysis) to either simvastatin (20 mg) plus ezetimibe (10 mg) daily, simvastatin (20 mg) daily, or placebo.6 After 1 year, patients in the simvastatin monotherapy arm were rerandomized to either the combination therapy or placebo cohorts.6
After a median follow-up period of 4.9 years, there was an average LDL difference of 0.85 mmol/L between the treatment and control groups.6 As compared with placebo, treatment with simvastatin-ezetimibe led to a 17% reduction in the incidence of major atherosclerotic events (P5.0021).6 Active treatment was also associated with significant reductions in nonhemorrhagic stroke (2.8% vs 3.8%, respectively; P5.01) and arterial revascularization procedures (6.1% vs 7.6%, respectively; P5.0036) compared with placebo.6 There were more patients in the treatment arm with hemorrhagic stroke compared with placebo (1% vs 0.8%, respectively; P5.4), however, these findings were nonsignificant.6 Treatment was not associated with an increased risk of myopathy, liver and biliary disorders, cancer, or nonvascular morality and had no effect on kidney disease progression, thereby enabling the researchers to conclude that the combination of simvastatin/ezetimibe safely reduces the incidence of major atherosclerotic events in a wide range of patients with CKD.6
Meta-analyses. Attempting to further clarify the effect of statins in patients with CKD, 2 recent meta-analyses examined the results of lipid-lowering therapy in this patient population.9,10 The first systematic review and meta-analysis evaluated randomized controlled trials conducted between January 2000 and November 2011 that compared lipid-lowering therapy (ie, statin therapy or statin plus ezetimibe) against a control in patients with CKD.9 Of the 18 trials included in the analysis, only 5 involved CKD populations whereas 13 included CKD subgroup analyses from trials conducted in the general population.9 The researchers reported that statin therapy significantly reduced cardiac mortality risk (pooled risk ratio from 6 trials, 0.82; P<.001), CV events including revascularization (pooled risk ratio from 9 trials, 0.78; P<.001), and MI (pooled risk ratio from 9 trials, 0.74; P<.001).9 Yet, little overall effect in dialysis patients was reported and no additional advantage was found for other CV outcomes, suggesting that the benefit of lipid-lowering therapy may be limited to a decrease in CV death and atherosclerosis-mediated CV events in patients not receiving hemodialysis.9
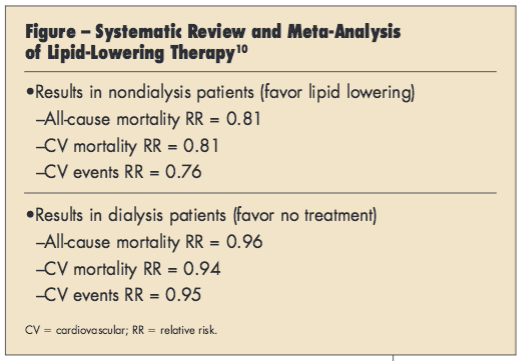
A second, larger meta-analysis included 80 randomized trials comparing the effects of statins with placebo, no treatment, or another statin on mortality and CV outcomes.10 The researchers reported that treatment effects varied by CKD stage, with statins found to decrease mortality and CV events in patients with early-stage CKD, but having had little or no effect in dialysis patients and uncertain effects in individuals with ESRD (Figure).10
GUIDELINES FOR SAFE AND EFFECTIVE DYSLIPIDEMIA MANAGEMENT IN PATIENTS WITH CKD
Given the strong association between CVD and CKD, particular attention toward factors that may mitigate CV risk (eg, smoking cessation, exercise, weight reduction, lipid modulation, glucose control) in patients with impaired kidney function is vital. Results of statin trials are mixed, yet there is a trend favoring modest benefit with lipid-lowering therapy, particularly for patients without ESRD or those not requiring hemodialysis.6,7-10 As such, it is essential for primary care clinicians—who are likely to care for patients with early-to-moderate stage CKD—to understand how to apply these findings in developing patient-centered treatment regimens for dyslipidemia management.
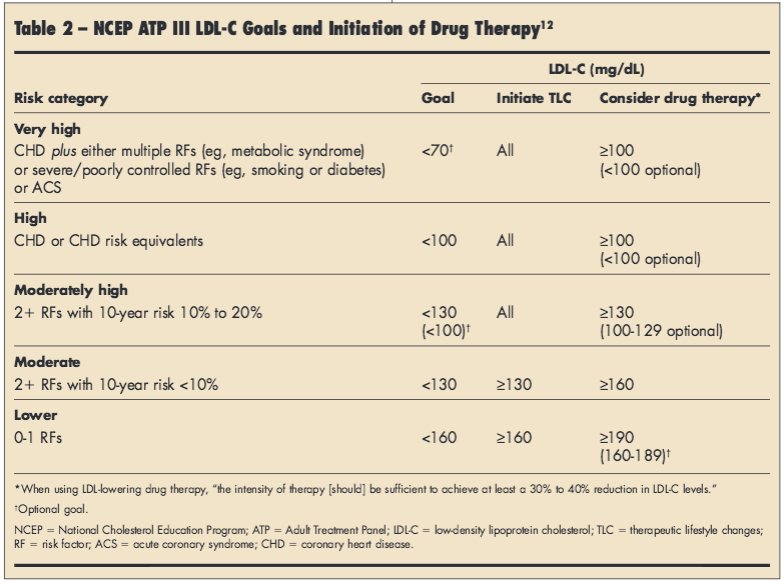
NCEP ATP III. Issued in 2002, the third report from the Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults of the National Cholesterol Education Program guidelines (NCEP ATP III) provides an algorithm for LDL targets versus risk.11 A 2004 update to ATP III outlines more aggressive targets for very high-risk persons and moderately high-risk individuals, but leaves low-risk individual targets unchanged (Table 2).12 TLC, including diet and exercise modifications, are stressed as therapy for all patients.11,12
Consulting these guidelines and conducting risk assessment in clinical practice is critical when determining appropriate treatment and can be simplified for easier application as follows12:
•Does the patient have established atherosclerotic CVD or diabetes?
–If yes, the patient should be considered at high- or very-high risk.
–If no, count the number of traditional risk factors.
•What is the patient’s number of traditional risk factors?
–If the patient has 0-1 traditional risk factors, then he/she is considered low risk.
–If the patient has 2 or more traditional risk factors, then he/she is at least moderate risk; risk can be further defined by calculating the Framingham Risk Score (FRS) for MI or coronary heart disease (CHD) death.
•What is the patient’s FRS?
–An FRS <10% is associated with moderate risk;
–An FRS ranging from 10% to 20% is associated with moderately high risk;
–And an FRS >20% is associated with high risk.
Changes to the ATP guidelines, tentatively titled ATP IV, are expected shortly and may lead to significant changes in risk assessment. In the interim, clinicians might further enhance global risk assessment to guide the intensity of lipid-lowering therapy by considering additional factors such as LDL particle size and number, high-density lipoprotein cholesterol (HDL-C) subtype and particle number, lipoprotein (a), homocysteine, apolipoproteins A1 and B, microalbuminuria, reduced GFR, N-terminal-pro-brain natriuretic peptide, high-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, carotid intima-medial thickness, coronary artery calcium score by computed tomography, and coronary computed tomography angiogram.13-18 Notably, these additional factors may serve to refine risk assessment, but should not substitute for global evaluation. Although it is likely that these supplementary considerations allow clinicians to treat to a more optimal target, data supporting this are largely lacking at present.
NCEP ATP III guidelines do not specifically include patients with CKD in the CHD risk equivalent group and make few specific recommendations regarding the evaluation and treatment of dyslipidemia in this patient population.11 Among the limited recommendations for the CKD population, the guidelines concede that chronic renal failure and nephrotic syndrome can cause secondary dyslipidemia and elevated triglycerides (TGs)19 and note that lipid-lowering therapy should be considered for CKD patients who experience persistent hyperlipidemia.11 The guidelines also acknowledge that, although dyslipidemias are common in patients with stage 5 CKD, clinicians should prescribe medical therapy with caution since patients with kidney failure are at increased risk for drug-related adverse effects including myopathy from statins and fibric acids.11,19
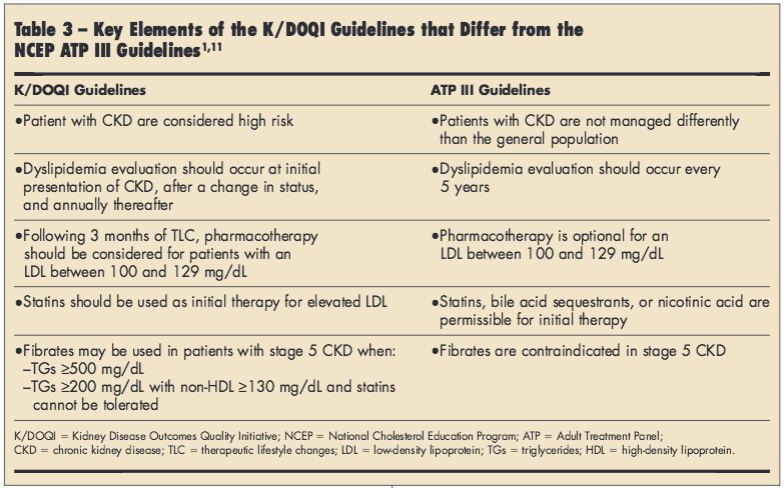
K/DOQI guidelines. Supplemental guidance for patients with CKD is provided in the K/DOQI recommendations released in 2003.1 These guidelines include an evidence-based algorithm for the management of dyslipidemia based on disease stage according to GFR.1 The guidelines specify that all adults and adolescents with CKD should be evaluated for dyslipidemia, including a complete fasting lipid profile with total cholesterol, LDL, HDL, and TGs; K/DOQI guidelines note that NCEP ATP III guidelines are applicable to patients with stages 1-4 CKD with a few modifications, but present separate recommendations for patients with stage 5 CKD.1 As these guidelines are intended to be employed in conjunction with ATP III recommendations, providers should be aware of key differences between the treatment algorithms to provide optimal dyslipidemia management in the setting of CKD (Table 3).1,11
INITIATING LDL-LOWERING THERAPY
Although lifestyle modifications are essential to lower LDL levels and reduce the risk for CHD, it is recognized by the ATP III and K/DOQI authors that TLC alone will not provide a sufficient LDL reduction to reach goal for most patients with concomitant CKD and dyslipidemia.1,11 Pharmacologic agents used to treat hypercholesterolemia include statins, a cholesterol uptake transporter inhibitor, bile acid sequestrants, niacin, fibrates, and omega-3 fish oils.20 While each drug class has a differing mechanism of action, in general they exhibit dose-dependent and complementary effects on LDL. Hence, guidelines indicate that, for the general population, treating to an LDL target can be accomplished either through dose-escalation monotherapy or through the additive effects of combination therapy.11
HMG-CoA reductase (statin) therapy. Statin therapy has been demonstrated to be effective in lowering LDL-C in certain stages of CKD.6,7-10 Whereas many patients can reach LDL-C goal with statin monotherapy, significant muscle-related adverse effects to commonly prescribed statins may prohibit dose escalation in some patients.21-26 For instance, in June 2011, the US Food and Drug Administration (FDA) recommended that simvastatin 80 mg should be used only in patients who have tolerated this dose for at least 12 months without evidence of myopathy.27 According to these recommendations, simvastatin 80 mg should not be started in new patients, including patients already taking lower simvastatin doses; patients who are not achieving adequate control on the 40 mg daily dose should be switched to a higher potency statin.27
Pharmacokinetic drug-drug and food-drug interactions have been identified as one contributing factor to such adverse drug reactions resulting from statin therapy. As such, the FDA also required a simvastatin labeling change to add new contraindications (ie, itraconazole, ketoconazole, posaconazole, erythromycin, clarithromycin, telithromycin, human immunodeficiency virus protease inhibitors, nefazodone, gemfibrozil, cyclosporine, danazol).27 In addition, simvastatin 10 mg daily should not be exceeded with verapamil or diltiazem, and simvastatin 20 mg daily should not be exceeded with amiodarone, amlodipine, or ranolazine.27
Although most patients with CKD will be able to reach LDL-C with statin monotherapy, some may require combination therapy either because their significant lipid abnormality does not allow for goal attainment with monotherapy or because of dose-limiting adverse effects. As such, combination lipid-lowering therapy may be indicated to reach goal. While statin monotherapy has been shown to reduce CV events in most patient population studies, the 2 available studies (ie, AURORA and 4D) that utilized this approach in CKD patients did not demonstrate a clinical benefit.7,8 The only study that has shown a clinical benefit with lipid-lowering therapy in a CKD population is the combination of a statin (ie, simvastatin) and ezetimibe.6 Whether this difference in result is due to the patient populations (ie, ESRD vs a wider group of subjects with CKD) or a unique effect of that particular combination is not known. No other combinations of lipid-lowering therapies have been extensively studied in patients with significant CKD, although this strategy may be considered in certain clinical scenarios.
Niacin. Patients with complex dyslipidemias may benefit from the addition of niacin to their treatment regimen, as this agent is recognized as effective for lowering LDL, raising HDL, lowering TGs, and increasing LDL particle size.28,29 There are no data at present, however, to support these effects in the CKD population. In addition, niacin can result in cutaneous flushing, although this may be minimized by choice of pharmacologic preparation and patient education/awareness on best practices of treatment.28,29 Dose-adjustments in patients with severe CKD or ESRD may be required.
Fibrates. In many patients with adequately controlled LDL but suboptimal HDL and TG levels, the addition of a fibrate to statin therapy may be a safe and effective option; however, guidelines point out there are very few data on the safety and efficacy of this combination in patients with CKD.1 As the mechanism of action for the interaction between these 2 classes of drugs is not well understood, it is suggested that fibrate-statin combination therapy be avoided for patients with advanced CKD. It is important to note, however, that fibrate monotherapy for the small, but not insubstantial, subset of patients who present with hypertriglyceridemia as their primary lipid abnormality is permissible and often considered first-line therapy in these instances.
Additional combination therapies. For those patients with elevated LDL despite TLC and optimal treatment with a statin, the addition of either a bile acid sequestrant or cholesterol transport inhibitor may augment the effectiveness of statin therapy in reducing LDL while minimizing statin-related adverse effects.1 Although evidence supports the use of bile acid sequestrants in the general population for lowering LDL by as much as 30%, no safety or efficacy data are available for the CKD population.1 In contrast, the combination of a statin and cholesterol transport inhibitor (ie, ezetimibe) was demonstrated in moderate-to-severe CKD to safely offer a reduction in major atherosclerotic events as described in the SHARP trial mentioned above.6 As such, given the overall paucity of data regarding combination therapy in this patient population, the choice of added agents should be dependent upon the individual’s comorbid conditions and full lipid profile after statin therapy.
OVERCOMING BARRIERS TO ADHERENCE
Patient adherence to prescribed therapy is a common problem in all therapeutic areas, and patients with CKD and dyslipidemia are no exception. A prospective study of 71 veterans given their first prescription of a statin for primary prevention found that at 6 months, approximately 50% of patients were nonadherent and 10% reported never having started their statin; among patients who discontinued statin use, median duration of use was less than 2 months.30 Poor adherence was related to several factors including perception of risk, toxic effects, expected treatment duration, and sociodemographic factors.30
As when treating any high-risk patient group, clinicians should identify strategies to enhance patient adherence to dyslipidemia therapy, given that nonadherence will diminish potential therapeutic benefit. Considerations include patient understanding about the need, safety, and effectiveness of therapy; specific patient concerns and misperceptions; financial and other access issues; and feedback from family and friends. The ESFT (Explanatory model, Social and financial risk for nonadherence, Fears and concerns about treatment, and Therapeutic contract) model of physician-patient communication incorporates several features to increase the likelihood of therapeutic adherence; it offers a contextualized explanation of the patient’s illness and treatment, evaluates social barriers to adherence, and encourages consensus between clinician and patient regarding treatment decisions.31
Other strategies for improving adherence to lipid-lowering therapy have been identified in the NCEP ATP III guidelines and include patient-centered, physician-centered, and system-centered interventions.19
Patient-centered interventions. Several patient-centered regimens have been proven to enhance adherence to drug therapy. These include simplifying medication regimens; providing clear instructions and using counseling techniques such as “teach back” to ensure that the patient understands how to follow the prescribed treatment regimen; encouraging the use of prompts to help patients remember to take their medicines at the appropriate times; encouraging patients to obtain the support of family and friends; praising adherence; increasing office visits for patients who are unable to achieve their treatment goal; and helping patients
develop monitoring strategies that enhance their self-management ability and commitment.19
Physician-centered interventions. Adherence interventions that focus on the clinician and medical office include developing systems to remind physicians to conduct lipid management activities; ensuring adequate training regarding lipid management goals, diagnosis, and treatments; identifying a patient advocate in the office to help deliver or prompt lipid evaluation and management; encouraging patients to inquire about lipid levels and management options; developing standardized treatment plans that incorporate lipid evaluation and management; and reminding patients of scheduled appointments.19
System-centered interventions. Interventions implemented at the healthcare delivery system level can also improve adherence to lipid-lowering therapy. For example, some systems have developed lipid clinics that manage this aspect of patient care. Other systems involve nurse case managers, pharmacists, registered dietitians, and other clinicians in educating patients about the importance of adherence.19 Knowledge of these available services can enable primary care providers to augment their patients’ treatment regimens and further promote adherence.
A PATIENT-CENTERED APPROACH TO LIPID MANAGEMENT: A CASE STUDY
MT is a 64-year-old African-American man with a long history of hypertension, but no history of diabetes or CVD. He has never been told that he has a problem with cholesterol, but has been informed previously that his kidney function is “a little off.” His medications include aspirin (81 mg daily), hydrochlorothiazide (25 mg daily), and ibuprofen, as needed. MT is a nonsmoker and denies regular alcohol use. He is making a first-time visit to a new primary care physician to establish care.
Upon physical examination, the physician determines that the patient’s blood pressure is 144/82 mm Hg and his heart rate is 72 bpm. Laboratory work reveals total cholesterol of 194 mg/dL, LDL of 128 mg/dL, HDL of 42 mg/dL, TGs of 132 mg/dL, serum creatinine of 1.9 mg/dL, estimated GFR of
38 mL/min/1.73 m2, and urine albumin-to-creatinine ratio of 162 mg/g; an electrocardiogram indicates normal sinus rhythm with left ventricular hypotrophy. The patient’s FRS is 16%.
Questions to consider:
•According to the current lipid guidelines, is this patient at high risk?
•In addition to TLC, is lipid-lowering medication
warranted?
•If the use of lipid-lowering therapy is warranted, which one or combination should be prescribed?
The physician notes that MT has stage 3B CKD with microalbuminuria and is at moderately high CVD risk per NCEP ATP III guidelines. Although he acknowledges that the probability of MT’s kidney disease progressing to ESRD is relatively high, the physician does not make a formal nephrology consult at this point since he usually reserves a referral for those patients with stage 4 CKD, rapid disease progression, or unknown etiology of CKD.
The physician tells MT that in addition to TLC, lipid-lowering medication (eg, a statin) is warranted. Lipid-lowering therapy is prescribed and the patient is scheduled for a 3-month follow-up visit.
Upon returning to the physician’s office 3 months later, MT’s lipid panel remains unchanged from baseline.
Questions to consider:
•What is the differential diagnosis?
•Is the patient resistant to the action of statin therapy?
Given that there was no change in MT’s lipid panel, the physician determines that his patient has not been taking the prescribed therapies. To address the adherence issues, the physician explains to MT the clinical consequences of not taking his medication as prescribed and questions him as to whether financial or other concerns have influenced his decision to be nonadherent. Before concluding the exam, the physician encourages MT to agree to another 3-month trial, letting him know that if he is not satisfied with the treatment by the time of his next appointment, they will work together to determine another therapeutic course of action. MT decides to try again, and both the physician and patient leave the exam optimistic about the mutually-agreed upon strategy.
CONCLUSION
The association between CKD and CVD is strong and convincing. CVD is the most common cause of mortality in patients with CKD, and the incidence of CV events correlates with the degree of kidney dysfunction. As such, primary care clinicians treating patients with CKD should consider the likelihood of co-occurring CVD and recommend risk reduction interventions related to smoking cessation, exercise, weight reduction, glucose control, and in particular, lipid modulation. Although results of clinical trials evaluating the effectiveness of statin therapy in improving CV outcomes in CKD patients are mixed, these studies reveal a trend toward benefits of treatment. Future research is needed to define a lipid-lowering strategy that optimizes safety, efficacy, and cost in patients with CKD; but in the interim, primary care providers have the potential to minimize clinical burden by monitoring and addressing factors known to increase CV risk.
Dr Bloch is Medical Director, Risk Reduction Center and Co-Director, Vascular Institute, St Mary’s Regional Medical Center, Reno, Nevada.
Dr Kuritzky is Clinical Assistant Professor, Family Medicine Residency Program, University of Florida, Gainesville, Florida.
REFERENCES:
1. K/DOQI Task Force. K/DOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease. Am J Kidney Dis. 2003;41(4 Suppl 3):S1-S92.
2. National Kidney Foundation. About Chronic Kidney Disease, 2012. https://www.kidney.org/kidneydisease/aboutckd. Accessed September 18, 2012.
3. Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17-28.
4. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
5. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL,et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney inCardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154-2169.
6. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in
patients with chronic kidney disease (Study of Heart and Renal Protection):a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. Epub 2011 Jun 12.
7. Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238-248.
8. Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395-1407.
9. Upadhyay A, Earley A, Lamont JL, Haynes S, Wanner C, Balk EM. Lipid-lowering therapy in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;157(4):251-262.
10. Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease:
a systematic review and meta-analysis. Ann Intern Med. 2012;157(4):263-275.
11. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
12. Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
13. Jones, PH. Clinical diagnosis of lipid disorders. Clin Cornerstone. 1998;1(1):15-30.
14. Kuller LH, Evans RW. Homocysteine, vitamins, and cardiovascular disease. Circulation. 1998;98(3):196-199.
15. Seman LJ, McNamara JR, Schaefer EJ. Lipoprotein(a), homocysteine, and remnantlike particles: emerging risk factors. Curr Opin Cardiol. 1999;14(2):
186-191.
16. Smith SC Jr., Greenland P, Grundy SM. AHA Conference Proceedings.Prevention conference V: Beyond secondary prevention: Identifying the high-risk patient for primary prevention: executive summary. American Heart Association. Circulation. 2000;101(1):111-116.
17. Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovasculardisease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med. 2000;160(8):
1093-1100.
18. Maeder MT, Mueller C, Pfisterer ME, Buser PT, Brunner-La Rocca HP. Use of B-type natriuretic peptide outside of the emergency department. Int J Cardiol. 2008;127(1):5-16.
19. Expert Panel on Detection, Evaluation, and Treatment of High BloodCholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
20. Toth PP. Drug treatment of hyperlipidaemia: a guide to the rational use of lipid-lowering drugs. Drugs. 2010;70:1363-1379.
21. Shanes JG. A Review of the rationale for additional therapeutic interventions to attain lower LDL-C when statin therapy is not enough. Curr Atheroscler Rep. 2012;14:33-40.
22. Hedenmalm K, Alvan G, Ohagen P, Dahl ML. Muscle toxicity with statins. Pharmacoepidemiol Drug Saf. 2010;19:223-231.
23. Backes JM, Howard PA, Ruisinger JF, Moriarty PM. Does simvastatin cause more myotoxicity compared with other statins? Ann Pharmacother. 2009;43:2012-2020.
24. Oshima Y. Characteristics of drug-associated rhabdomyolysis: analysis of 8,610 cases reported to the U.S. Food and Drug Administration. Intern Med. 2011;50:845-853.
25. Whayne TF Jr. Statin myopathy: significant problem with minimal awareness by clinicians and no emphasis by clinical investigators. Angiology. 2011;62:415-421.
26. Davignon J. Pleiotropic effects of pitavastatin. Br J Clin Pharmacol. 2012;73:518-535.
27. Food and Drug Administration. FDA drug safety communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Accessed October 18, 2012.
28. McKenney JM. Combination treatment with atorvastatin plus niacin provides effective control of complex dyslipidemias: a literature review. Postgrad Med. 2012;124:7-20.
29. Toth PP, Thakker KM, Jiang P, Padley RJ. Niacin extended-release/simvastatin combination therapy produces larger favorable changes in high-density
lipoprotein particles than atorvastatin monotherapy. Vasc Health Risk Manag. 2012;8:39-44.
30. Mann DM, Allegrante JP, Natarajan S, Halm EA, Charlson M. Predictors of adherence to statins for primary prevention. Cardiovasc Drugs Ther. 2007;21(4):
311-316.
31. Bloch MJ, Betancourt J, Green A. Overcoming racial and ethnic disparities in blood pressure control: a patient-centered approach to cross-cultural communication. J Clin Hypertens (Greenwich). 2008;10(8):589-591.


