Peer Reviewed
A Collection of Cases of Sweet Syndrome
Sweet Syndrome Associated With Acute Myeloid Leukemia
Authors:
Hendy B. Jean, MD; Htet Htet Win, MD; Sabita Sarkar, MD; Minale Desta, MD; Ashhar Ahmed, MS-III; and Harish Patel, MD
Kingsbrook Jewish Medical Center, Brooklyn, New York
Citation:
Jean HB, Win HH, Sarkar S, Desta M, Ahmed A, Patel H. Sweet syndrome associated with acute myeloid leukemia. Consultant. 2017;57(5):300-301.
A 56-year-old man presented to our emergency department (ED) with fever, chills, dyspnea, diarrhea, and vomiting. The patient had been in his usual state of normal health until 2 weeks prior, when he had visited a different ED for a lump in his left buttock. The lump was described as a red-to-purple nodule that was nonfluctuant and without crepitation. He had been discharged home on a regimen of oral ciprofloxacin and metronidazole.
One week thereafter, the lesion had not improved, and the patient presented to another health care facility where incision was performed without drainage of pus.
History and physical examination. On presentation to our ED, the patient reported having fever, chills, dyspnea, diarrhea, and vomiting but denied cough, sputum production, sick contacts, or recent travel. On physical examination, he appeared lethargic and was febrile (temperature, 38.9°C), with a blood pressure of 152/92 mm Hg and a heart rate of 102 beats/min.
Skin examination showed a red-to-purple lesion in the left buttock (Figure 1) without fluctuation or crepitation, as well as 3 new lesions on the left forehead (Figure 2), the left tragus (Figure 3), and the right side of the upper lip. Cardiovascular examination findings were remarkable for tachycardia and an irregular pulse. The rest of the physical examination findings, including examination of the lungs, abdomen, rectum, and extremities, were unrevealing.

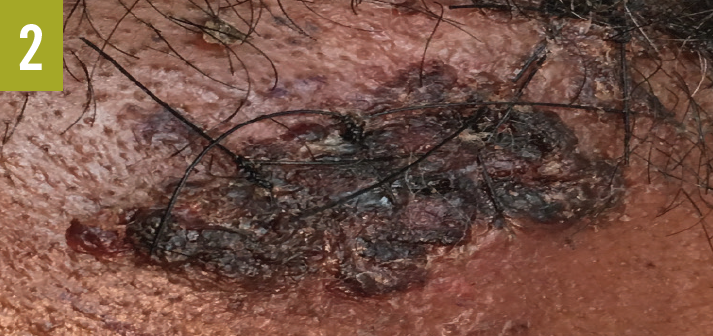
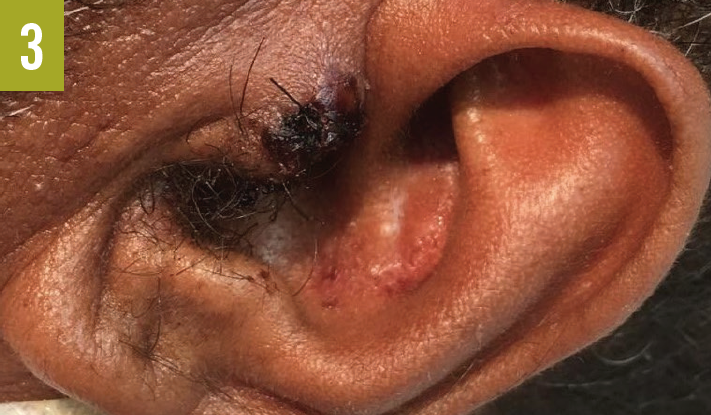
Diagnostic tests. Results of initial laboratory studies were notable for a white blood cell count of 29,700/µL, a blood urea nitrogen level of 52 mg/dL, and a creatine level of 1.5 mg/dL. Electrocardiography showed atrial fibrillation with rapid ventricular response. Chest radiography revealed no acute chest pathology. Computed tomography scanning of the abdomen revealed no acute intra-abdominal process, abscess, or collection but did show soft tissue swelling of the left buttock without abscess or collection.
The patient initially was treated for presumed sepsis with broad-spectrum antibiotics. Due to the severity and complexity of the disease on presentation, clinicians from multiple specialties (including cardiology, infectious disease, gastroenterology, and general surgery) were involved in the management of the patient’s condition. Pan-culture test results remained negative for pathogens throughout his entire hospital stay, as did the results of stool DNA tests for Clostridium difficile.
Another incision performed at the site of the left buttock lesion revealed no abscess or pus collection. His diarrhea had been persistent and unresponsive to antibiotics and antidiarrheal therapy. Findings of a colonoscopy performed by the gastroenterologist ruled out inflammatory bowel disease. Despite treatment, the leukocytosis, skin lesions, and general condition of the patient worsened, prompting consultation with specialists in hematology-oncology and dermatology.
Diagnosis. Based on the results of bone marrow biopsy, flow cytometry testing, and skin biopsy of the lesions on his left forehead, left tragus, and left buttock, the patient received a diagnosis of acute myeloid leukemia (AML) associated with Sweet syndrome.
Discussion. Sweet syndrome, also called acute febrile neutrophilic dermatosis, is an uncommon inflammatory disorder characterized by the abrupt onset of painful dermatologic lesions. Its presentation ranges from classic Sweet disease, which can occur after mild respiratory illness, to a more aggressive neutrophilic process, which may be associated with other inflammatory diseases or malignancies, especially hematologic malignancies.
The initial presentation of the patient pointed more toward an infectious process; however, as data from the history, physical examination, laboratory studies, imaging studies, and his response to therapy during the hospital course were collected, the clinical picture increasingly pointed toward a more intricate and complicated disease process.
Among the differential diagnoses is pyoderma gangrenosum, which can present in a similar fashion to Sweet syndrome with constitutional symptoms, gastrointestinal tract symptoms in association inflammatory bowel disease, and skin lesions. The skin biopsy report in our patient’s case ruled out pyoderma gangrenosum, however.
Outcome of the case. Once the diagnosis of Sweet syndrome in association with AML had been established, the patient was started on prednisone therapy, which led to dramatic improvement in the skin lesions. Further therapy, including chemotherapy, eventually resulted in resolution of all the man’s physical symptoms as well as the neutrophilia. He was discharged in stable condition after being transferred to another facility for chemotherapy, and a plan for follow-up was made with his oncologist.
NEXT: Sweet Syndrome Associated With Cocaine Use
Sweet Syndrome Associated With Cocaine Use
Authors:
Samuel Slone, BS, and Igor Sunjic, MD
University of South Florida Morsani College of Medicine, Tampa, Florida
Citation:
Slone S, Sunjic I. Sweet syndrome associated with cocaine use. Consultant. 2017;57(5):301-303.
A 37-year-old woman with a history of intravenous hydromorphone use and crack cocaine use presented to the emergency department with a 1-day history of a pustular rash associated with burning pain.
History. The pustular lesions had begun on her hands bilaterally and had quickly progressed over her upper extremities, chest, back, abdomen, lower extremities, and face over the course of 24 hours, without mucosal involvement. She denied fever, joint pain, chest pain, abdominal pain, dyspnea, or recent illnesses. She stated that she had taken a 4-day course of amoxicillin prior to admission for a self-diagnosed ankle infection. She had received the antibiotics from a friend. She reported having had a similar rash 1 year prior to presentation that had been localized to her posterior calves and had occurred after the use of clindamycin for an unknown infection.
She initially presented to an outside hospital and had been given vancomycin, piperacillin-tazobactam, and acyclovir out of concern for widespread infection. She had been transferred to our facility for further evaluation.
Physical examination. The patient was an uncomfortable-appearing woman with a diffuse, widespread pustular rash. She was febrile (temperature, 39.4°C) and had a heart rate of 120 beats/min. The rash involved the bilateral hands, upper and lower extremities, and feet (Figures 1-3), as well as the face, neck, and superior chest. There was minimal involvement of the posterior lower extremities and posterior trunk. Scattered pustules were present on the abdomen and labia. Vesicular lesions were present on the right volar distal forearm, and larger ulcerated lesions were present on the dorsal hands and forearms (Figure 4), with a positive Nikolsky sign. There were no lesions in the oral mucosa or on the palms or soles.



Diagnostic tests. The initial white blood cell (WBC) count was 8570/µL with a neutrophilic predominance (72%). The C-reactive protein (CRP) level was 25.21 mg/L, and the erythrocyte sedimentation rate (ESR) was 53 mm/h. Blood, fungal, wound, anaerobic, and acid-fast cultures were taken, and all were without growth throughout admission. Results of polymerase chain reaction tests for varicella-zoster virus and herpes simplex virus were negative. The results of all other initial laboratory tests, including a complete blood cell count (CBC) and reticulocyte count, were unremarkable.
Results of a skin biopsy of the left thigh showed a wedge-shaped lesion with partial epidermal necrosis, blister formation, and diffuse neutrophilic infiltrate, with nuclear fragmentation in the upper portion of the reticular dermis (Figures 5 and 6). The histologic findings had the combined features of Sweet syndrome, also known as acute febrile neutrophilic dermatosis.

Discussion. Sweet syndrome is an uncommon inflammatory disorder characterized by the abrupt appearance of burning, painful, erythematous papules, plaques, and cutaneous nodules that are typically accompanied by fever and leukocytosis with neutrophil predominance.1 Sweet syndrome is typically subdivided into 3 categories: classic, malignancy-associated, and drug-induced.
While most cases of classic Sweet syndrome are idiopathic, cases have been linked to infections (particularly in the upper respiratory and gastrointestinal tracts), inflammatory bowel disease, and pregnancy.1 There have also been less-documented associations with autoimmune conditions, primary immunodeficiency, and other infections (eg, human immunodeficiency virus, tuberculosis).
Malignancy-associated Sweet syndrome accounts for approximately 21% of cases.2 A stronger association exists with hematologic malignancies than with solid tumor cancers.3 Acute myeloid leukemia has the strongest association with Sweet syndrome, accounting for approximately 42% of associated hematologic malignancies.4
Drug-induced Sweet syndrome has been linked to the use of a variety of illicit drugs and medications. Notably, it has been linked to the use of cocaine. In terms of medications, it is most strongly associated with granulocyte-colony stimulating factor,5 and the condition has also been linked to antibiotics, nonsteroidal anti-inflammatory drugs, and antineoplastic agents.
In order to receive a diagnosis of Sweet syndrome, a patient must exhibit the 2 major criteria: abrupt onset of painful, erythematous plaques or nodules, and histopathologic evidence of a dense, neutrophilic infiltrate without evidence of leukocytoclastic vasculitis.6 The patient must also exhibit 2 of 4 minor criteria: pyrexia above 38°C; association with an underlying hematologic or visceral malignancy, inflammatory disease, or pregnancy, or preceded by an upper respiratory tract or gastrointestinal tract infection or vaccination; excellent response to treatment with systemic corticosteroids or potassium iodide; and abnormal laboratory values at presentation (3 of the following 4: ESR greater than 20 mm/h, elevated CRP; a WBC count above 8000/µL, or a differential cell count showing greater than 70% neutrophils).6 Our patient met these diagnostic criteria and, coupled with the overall clinical picture, a diagnosis of Sweet syndrome was confirmed.
Outcome of the case. The patient was placed on 60 mg of oral prednisone daily, with a planned 6-week taper. Over the first 48 hours after medication administration, the patient’s symptoms demonstrated significant improvement. She continued to improve on the corticosteroid taper.
It was believed that the inciting event for the development of Sweet syndrome was either idiopathic or was related to the patient’s use of cocaine. Given that her initial CBC and reticulocyte count findings were unremarkable, further workup for acute myeloid leukemia was not pursued.
REFERENCES:
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Cohen PR, Kurzrock R. Sweet’s syndrome and cancer. Clin Dermatol. 1993;11(1):149-157.
- Raza S, Kirkland RS, Patel AA, Shortridge JR, Freter C. Insight into Sweet’s syndrome and associated-malignancy: a review of the current literature. Int J Oncol. 2013;42(5):1516-1522.
- Cohen PR, Talpaz M, Kurzrock R. Malignancy-associated Sweet’s syndrome: review of the world literature. J Clin Oncol. 1988;6(12):1887-1897.
- White JML, Mufti GJ, Salisbury JR, Du Vivier AWP. Cutaneous manifestations of granulocyte colony-stimulating factor. Clin Exp Dermatol. 2006;31(2):206-207.
- von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31(4):535-556.
NEXT: Sweet Syndrome Associated With Essential Thrombocythemia Transformation to Acute Myeloid Leukemia
Sweet Syndrome Associated With Essential Thrombocythemia Transformation to Acute Myeloid Leukemia
Authors:
Rita Raturi, MD
University of South Florida Morsani College of Medicine, Tampa, Florida
Charlotte O’Leary, MD, and Sudeep Gaudi, MD
James A. Haley Veterans’ Hospital, Tampa, Florida
Citation:
Raturi R, O'Leary C, Gaudi S. Sweet syndrome associated with essential thrombocythemia transformation to acute myeloid leukemia. Consultant. 2017;57(5):303-304.
A 62-year-old man presented with new-onset, erythematous lower-extremity skin lesions (Figure 1), which he had discovered after having changed a flat tire on gravel. He had a medical history of JAK2-negative essential thrombocythemia (ET), for which he had been taking hydroxyurea; type 2 diabetes; and thromboembolic disease, for which he was on systemic anticoagulation with warfarin. Of note, he also was being treated for progressively worsening anemia and thrombocytopenia, for which his hydroxyurea had been held as an outpatient.

The patient’s lesions markedly improved with the administration of intravenous broad-spectrum antibiotics (cefepime and vancomycin). He was discharged to home on a 7-day course of minocycline for a suspected diagnosis of localized posttraumatic cellulitis. Minocycline was chosen because of the need for methicillin-resistant Staphylococcus aureus coverage due to its high prevalence in our area. Doxycycline causes increased photosensitivity in 20% of patients compared with minocycline,1 which is noted to rarely cause photosensitivity, thus making it ideal for our patient, who is frequently outdoors.
However, the patient returned 2 days later with similar nodular lesions located diffusely on his body (Figures 2 and 3), as well as worsening fevers of up to 38.9°C.

Diagnostic tests. Laboratory test results revealed increasing leukocytosis from 11,000 to 20,000 cells/µL with elevated blasts, a decreased hemoglobin from 9.0 to 7.0 g/dL, and a decreased platelet count from 100 × 103/µL to 60 × 103/µL. The C-reactive protein (CRP) level was 1.6 mg/dL, and the erythrocyte sedimentation rate (ESR) was 90 mm/h.
A hematologist was consulted for additional assistance given the patient’s leukocytosis, anemia, and thrombocytopenia. An infectious disease consultant proceeded to monitor the patient without additional antimicrobial coverage, because the condition was likely not infectious.
Results of a peripheral blood smear revealed teardrop cells, nucleated red blood cells, and giant platelets. Results of a workup for disseminated intravascular coagulation were negative. An antinuclear antibody test and complement testing were ordered for further workup of an autoimmune etiology; however, the results were unrevealing. Flow cytometry studies revealed an increased proportion of phenotypically abnormal, immature myeloid blasts and immunophenotypic abnormalities of the myeloid lineage.
A dermatologist was consulted for further recommendations given the widespread cutaneous involvement. The results of a skin biopsy (Figure 4) of one of the lesions showed Sweet syndrome, also known as acute febrile neutrophilic dermatosis. A subsequent bone marrow biopsy confirmed transformation to acute myeloid leukemia (AML) from ET.
Discussion. Classically, the neutrophilic lesions of Sweet syndrome have accompanying papillary edema, as first described by R. Douglas Sweet, MD, in 1964.2 Commonly misdiagnosed as a soft tissue infection, Sweet syndrome is defined by an abrupt onset of painful or erythematous plaques or nodules; pyrexia; histologic evidence of dense neutrophilic infiltrate without vasculitis; a response to corticosteroid therapy; and an association with hematologic or visceral malignancy (in approximately 21% of cases).1 The condition may be preceded by a respiratory or gastrointestinal tract infection or a vaccination.
The diagnostic criteria also include 3 of 4 of the following: an ESR greater than 20 mm/h, an elevated CRP, a white blood cell count above 8000/µL, or a differential cell count showing greater than 70% neutrophils.3 Our patient met all 4 of these diagnostic criteria.
Other underlying disease processes, in addition to hematologic or solid tumor malignancy, include inflammatory bowel disease and pregnancy. Drug-induced Sweet syndrome also has been well documented in the literature and is associated with granulocyte-colony stimulating factor, all-trans retinoic acid, and other miscellaneous drugs in adults.4,5
Systemic corticosteroids are the gold standard treatment, with the ideal prednisone dose being 1 mg/kg daily with a slow taper over 4 to 6 weeks.3 Other pharmacotherapies include anakinra, clofazimine, indomethacin, dapsone, or colchicine if a corticosteroid-sparing agent is needed.3,4 Approximately one-third of patients who develop Sweet syndrome will experience a reoccurrence.3
Outcome of the case. Our patient received 60 mg prednisone once daily for 1 month, which resulted in the complete resolution of the cutaneous symptoms. He began outpatient chemotherapy for AML.
REFERENCES:
- Vassileva SG, Mateev G, Parish LC. Antimicrobial photosensitive reactions. Arch Intern Med. 1998;158(18):1993-2000.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76(8-9):349-356.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Kluger N, Gil-Bistes D, Guillot B, Bessis D. Efficacy of anti-interleukin-1 receptor antagonist anakinra (Kineret®) in a case of refractory Sweet’s syndrome. Dermatology. 2011;222(2):123-127.
- Kim MJ, Choe YH. Eponym: Sweet syndrome. Eur J Pediatr. 2010;169(12):1439-1444.


