Peer Reviewed
Challenging the Diagnosis of Asthma With Methacholine
Authors:
Florence Chau-Etchepare, MD; Robert M. Bayliss Jr, RCP, RRT; and Samuel Louie, MD
Citation:
Chau-Etchepare F, Bayliss RM Jr, Louie S. Challenging the diagnosis of asthma with methacholine. Consultant. 2018;58(9):242-244.
Asthma remains widely perceived as a homogenous disorder caused by allergic (extrinsic) or nonallergic (intrinsic) triggers. A new patient with a history of hay fever and past cigarette use has wheezing, a productive cough, and dyspnea on exertion—it must be asthma. Or is it?
A common pitfall is to treat asthma before determining whether the patient really has asthma. Unlike in clinical trials, practical experience (ie, mistakes in clinical practice) has shown that asthma is difficult to confirm without objective pulmonary function testing. Many patients with cough, dyspnea, and wheezing have been labeled as having asthma but actually have other conditions that go unrecognized. It is important to remember that when meeting a patient with this diagnosis but with no confirmatory testing, it is our duty to ask: Is it really asthma? It is ill-advised and even unsafe to prescribe asthma medications before confirmatory diagnostic testing.
People with asthma have expiratory airflow limitation from bronchospasm that leads to air-trapping and lung hyperinflation. Symptoms can be intermittent or persistent, with poorly controlled asthma leading to worse quality of life, absenteeism from work or school, and significant risk of acute exacerbations. Morbidity and mortality are significantly higher in persons with poorly controlled asthma. Expiratory airflow limitation can be detected by pulmonary function testing, but 50% to 60% of patients with clinically diagnosed asthma never receive such objective testing.1,2 Why pulmonary function testing is not being used more often to confirm asthma may reside in the experience of clinicians and the administrative and electronic documentation demands that create barriers to clinical reflection and more thoughtful assessment of patients.
The triad of wheezing, cough, and dyspnea on exertion are nonspecific for asthma but may be responsible for its overdiagnosis, which can lead to inappropriate prescribing of albuterol and high-dose inhaled and/or oral corticosteroids. Some patients continue on inhaled or systemic corticosteroids for long periods without a correct diagnosis or indication and are thus exposed to the long-term consequences of these medications.
Gastroesophageal reflux disease (GERD) and postnasal drip from allergic or nonallergic rhinosinusitis were the most common among 22 alternative diagnoses in 213 of 613 adult participants in 1 study.1 In the study, physician-diagnosed asthma was established within the past 5 years in Canadian cities from January 2012 to February 2016. The diagnosis of asthma was refuted in 33.1% of cases. Refuting asthma diagnoses through confirmatory testing gives clinicians and patients the confidence to safely stop asthma medications when there is no evidence of airflow obstruction on spirometry, no evidence of bronchial airway hyperresponsiveness (AHR), and no worsening of asthma symptoms after having all asthma medications tapered off.1
New insights have advanced our understanding that asthma is a clinical syndrome in a spectrum of several phenotypes with overlapping if not redundant chronic inflammatory cytokine profiles. Chronic airway inflammation leads to persistent airway obstruction from abnormal or exaggerated bronchial smooth-muscle constriction, mucus production, and airway edema.
However, bronchial AHR remains the characteristic pathophysiologic feature of all asthma syndromes and is not equivalent to postbronchodilator reversibility as defined by the Global Initiative for Asthma (GINA) 2018 report (at least 12% reversibility and at least 200 mL [or 400 mL] improvement in forced expiratory volume in the first second of expiration [FEV1]).3 The parasympathetic nervous system with its muscarinic receptors is agonized by the neurotransmitter acetylcholine and abnormal AHR. Patients who do not have asthma can be reliably identified by the absence of bronchial AHR with a negative methacholine challenge test (MCT) (provocative concentration causing a 20% or more decrease in FEV1 [PC20]) (Table 1).

We reviewed the indications and contraindications for MCT, or bronchial provocation testing, and its interpretation that can help refute asthma as a diagnosis.4,5
NEXT: Methacholine Challenge
METHACHOLINE CHALLENGE
Methacholine is a synthetic analogue of acetylcholine, the neurotransmitter for the parasympathetic nervous system innervating the airways. Methacholine is a pregnancy category C drug. Albuterol, used for reversal of bronchoconstriction caused by methacholine, also is in pregnancy category C.
The parasympathetic nervous system innervates the lower respiratory tract smooth muscles that surround bronchial airways. Nerve endings release acetylcholine to stimulate M3 muscarinic receptors found on bronchial smooth muscle and ultimately lead to muscle contraction, mucus hypersecretion, and airways constriction. Methacholine will provoke bronchoconstriction, symptoms of cough and wheezing, and a fall in baseline FEV1 in a similar manner. Everyone eventually will respond to methacholine in this manner, but much less methacholine is needed in people with asthma to reduce FEV1. In addition to methacholine, inhaled dry air, inhaled histamine, inhaled adenosine, inhaled mannitol, and leukotriene E4 have been reported to lead to acute decrease in FEV1.6,7
Unlike cyclic adenosine monophosphate, methacholine does not cause mast cells to degranulate, releasing neutrophil chemotactic factors.6 Unlike mannitol in a handheld dry powder inhaler, methacholine does not causes changes in bronchial mucosal osmolarity and the subsequent release of inflammatory mediators from mast cells.7
MCT PROCEDURE
MCT is performed at UC Davis Medical Center after informed consent by pulmonary technicians, typically registered respiratory therapists.
Spirometry is performed before methacholine administration to establish baseline and after each consecutive dose of methacholine. The patient inhales methacholine aerosol in increasing concentrations (mg/mL). After each dose of methacholine is administered, spirometry is performed again (forced vital capacity [FVC] maneuver), and FEV1 is recorded until the following occur: (1) the last or maximum dose of methacholine has been administered (16 mg/mL); (2) FEV1 has decreased 20% or greater from baseline; or (3) termination by staff due to patient fatigue, intolerance, signs of respiratory distress, stridor, or the patient refusing to continue testing.
Results are reported as a percent decrease in FEV1 from baseline spirometry. At UC Davis, the protocol is a modified dosing schedule from the American Thoracic Society, increasing from 0.5 mg/mL to 1, 2, 4, 8, and 16 mg/mL, the latter of which is the last and maximum dose recommended.5
The maximum dose of methacholine can vary among pulmonary function testing laboratories. For example, the Mayo Clinic protocol begins with a dose of 5 or 25 mg/mL.8 Three minutes later, FVC is done, and if the FEV1 has not declined by 20% or more, 4 additional breaths of 25 mg/mL are delivered. Three minutes later, FVC is done again; if no decline in FEV1 of 20% or more is observed, the test results are interpreted as negative. A positive result is defined as a 20% decrease in FEV1 induced by methacholine.
Albuterol for nebulization, ipratropium bromide for nebulization, and epinephrine 1:1,000 dilution (0.3 mg in adults, 0.15 mg in children) for intramuscular injection must be available in the pulmonary function laboratory to reverse severe bronchospasm.
NEXT: Test Interpretation
TEST INTERPRETATION
A positive MCT result is confirmed with a 20% or greater fall in FEV1 from baseline spirometry between 0.5 mg/mL and 16 mg/mL. The dose concentration at which a positive MCT (PC20) occurs is reported, indicating the level of AHR. A PC20 range of 8 to 16 mg/mL is considered clinically significant enough to separate patients with asthma from those without asthma (Figures 1 and 2).
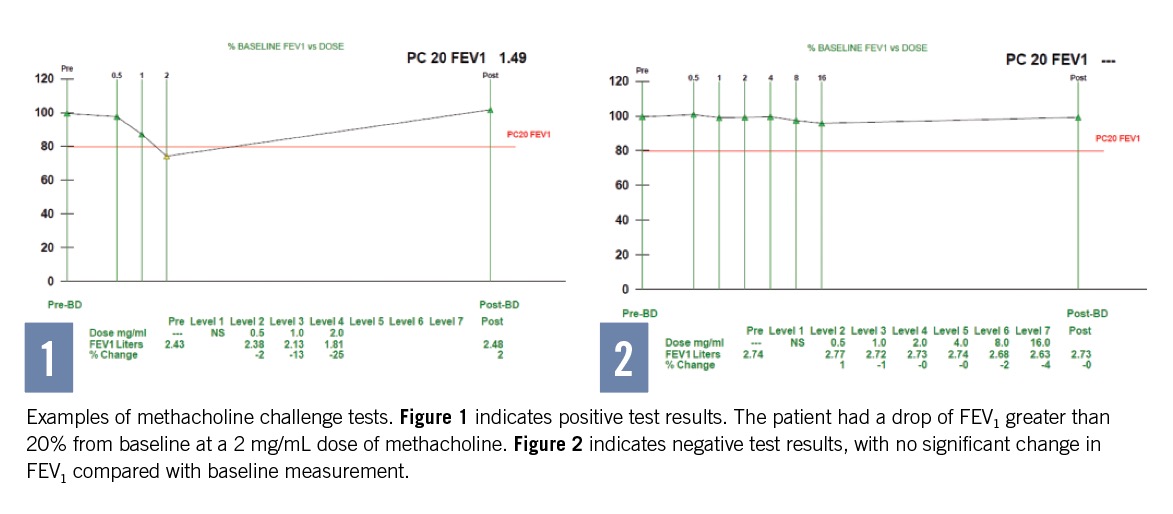
With a positive test result, signs and symptoms are recorded and nebulized albuterol is administered. After waiting 10 minutes, spirometry is performed to confirm that FEV1 has returned to at least 85% of baseline. If the FEV1 does not fall by at least 20% with the highest methacholine concentration, the test results are considered negative.
POTENTIAL COMPLICATIONS DURING MCT
It is a pitfall not to pursue MCT to detect bronchial AHR and challenge the diagnosis of asthma. Complications are uncommon. However, severe bronchospasm can occur, mimicking acute severe asthma, including lung hyperinflation and hypoxemia from ventilation and perfusion mismatching. Physicians are available to treat acute bronchospasm and use resuscitation equipment in the event of severe complications such as acute respiratory failure. Pulmonary laboratory technicians and clinicians must be trained and prepared to treat and manage these patients. Dizziness, lightheadedness, near syncope, hypertension, or sinus tachycardia greater than 120 beats/min can occur. There is no increased risk of cardiac arrhythmias during MCT.9
NEXT: Summary
SUMMARY
It is a pitfall to continue asthma medications in patients with no evidence of airflow obstruction on spirometry, no evidence of bronchial AHR, or no objective evidence of benefit from taking asthma medications (ie, relief of symptoms, improvement in Asthma Control Test score). AHR is defined as a decrease in airway expiratory flow or an increase in airflow limitation in response to a bronchoconstrictor (eg, dry air, histamine, methacholine, adenosine, mannitol, leukotriene E4). AHR is most commonly detected by methacholine.5
A baseline FEV1/FVC ratio of less than 0.70 defines airway obstruction, and MCT may not be indicated.
A negative MCT result rules out asthma in patients who have had asthma symptoms. When there is a moderate to high clinical suspicion of asthma, a negative MCT result has a greater than 90% negative predictive value. False negatives can arise if AHR has been suppressed by drug treatment; if MCT is performed at a time and place where seasonal allergy triggers are reduced; and if asthma is triggered by a single occupational antigen or chemical.5,10
A positive MCT result simply confirms the presence of bronchial AHR. A positive test result for bronchial AHR can be provoked in patients with asthma, as well as patients with chronic obstructive pulmonary disease (COPD), asthma-COPD overlap, GERD, vocal cord dysfunction, allergic rhinosinusitis, pulmonary sarcoidosis, chronic bronchiectasis, and after upper respiratory tract infection. A positive test result does not imply that asthma is also present in these other conditions.

Florence Chau-Etchepare, MD, is senior fellow in the Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine at UC Davis Health in Sacramento, California.
Robert M. Bayliss Jr, RCP, RRT, is a pulmonary laboratory technician IV at UC Davis Medical Center in Sacramento, California.
Samuel Louie, MD, is a professor of medicine in the Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine and is the director of the UC Davis Asthma Network (UCAN) at UC Davis Health in Sacramento, California.
REFERENCES:
- Aaron SD, Vandemheen KR, FitzGerald JM, et al; Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317(3):269-279.
- Gershon AS, Victor JC, Guan J, Aaron SD, To T. Pulmonary function testing in the diagnosis of asthma: a population study. Chest. 2012;141(5):1190-1196.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2018 Update). https://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed August 21, 2018.
- Coates AL, Wanger J, Cockcroft DW, et al. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J. 2017;49(5):1601526.
- American Thoracic Society. Guidelines for methacholine and exercise challenge testing—1999. Am J Respir Crit Care Med. 2000;161(1):309-329.
- Polosa R, Rorke S, Holgate ST. Evolving concepts on the value of adenosine hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Thorax. 2002;57(7):649-654.
- Anderson SD, Brannan J, Spring J, et al. A new method for bronchial-provocation testing in asthmatic subjects using a dry powder of mannitol. Am J Respir Crit Care Med. 1997;156(3 pt 1):758-765.
- Hahn PY, Morgenthaler TY, Lim KG. Use of exhaled nitric oxide in predicted response to inhaled corticosteroids for chronic cough. Mayo Clin Proc. 2007;82(11):1350-1355.
- Malerba M, Radaeli A, Politi A, Ceriani L, Zulli R, Grassi V. Cardiac arrhythmia monitoring during bronchial provocation test with methacholine. Chest. 2003;124(3):813-818.
- Gilbert R, Auchincloss JH Jr. Post-test probability of asthma following methacholine challenge. Chest. 1990;97(3):562-565.


