Peer Reviewed
Nitrous Oxide Use–Induced Vitamin B12 Deficiency With Subacute Combined Degeneration of the Spinal Cord
AUTHORS:
Navneet Attri, MD1,2 • Norick Jahingiri Janian, MD1
AFFILIATIONS:
1Sutter Santa Rosa Regional Hospital, Santa Rosa, California
2Pacific Inpatient Medical Group, Santa Rosa, California
CITATION:
Attri N, Janian NJ. Nitrous oxide use–induced vitamin B12 deficiency with subacute combined degeneration of the spinal cord. Consultant. 2020;60(12):18-20. doi:10.25270/con.2020.06.00005
Received February 22, 2020. Accepted May 7, 2020.
DISCLOSURES:
The authors report no relevant financial relationships.
CORRESPONDENCE:
Navneet Attri, MD, Sutter Santa Rosa Regional Hospital, 30 Mark West Springs Rd, Santa Rosa, CA 95403 (attrin@sutterhealth.org)
A 49-year-old man presented to our emergency department with a 1-day history of progressive bilateral lower-extremity weakness and an inability to bear weight.
The patient had been diagnosed with influenza 4 days prior and had been prescribed oseltamivir after having experienced high fevers with chills and generalized myalgias but no sore throat, cough, or coryza. He reported improvement in his influenza symptoms until 1 day prior, when he had noted tingling and weakness in his legs. He noted his legs to be “asleep” and numb. When he had tried to stand up, he buckled down on his knees and could not pull himself up to standing.
History. His medical history was remarkable for type 2 diabetes (which was controlled on insulin glargine, with a hemoglobin A1c of 6.1%), hypertension (for which he was on lisinopril), and hyperlipidemia. He had quit smoking. He had had “an alcohol problem” but had quit drinking 5 years ago after an isolated attack of pancreatitis. He reported smoking marijuana daily. He was married and had 3 children.
Physical examination. The patient had a body mass index of 28 kg/m2, was nontoxic appearing, and was emotionally labile, which he attributed to “panic” from the white coat effect. Cranial nerve function was normal. The lower extremities demonstrated profound ataxia, with no sense of position. Sensations were diminished to light touch. Plantar reflexes were abnormal, producing extension of the great toe instead of flexion. Knee reflexes were normal, and Achilles deep-tendon reflexes appeared brisk. Upper extremity reflexes were normal.
Diagnostic tests. Laboratory test results indicated leukopenia (white blood cell count, 3200/µL) with neutropenia (absolute neutrophil count, 1800/µL). The hemoglobin level was 13.1 g/dL with a mean corpuscular volume of 93 µm3, mean corpuscular hemoglobin of 32 pg/cell, and mean corpuscular hemoglobin concentration of 35 g/dL. The creatine kinase level was normal at 102 U/L.
Findings of magnetic resonance imaging (MRI) of the brain were unremarkable except for an elongated 1.8-cm pineal cyst in the anteroposterior dimension. The differential diagnosis at this point was post-influenza transverse myelitis versus Guillain-Barré syndrome. Lumbar puncture and MRI of the thoracic spine were ordered.
Lumbar puncture revealed colorless cerebrospinal fluid with 1 white blood cell, 6 red blood cells, a glucose level of 45 mg/dL (the serum glucose level at the time was 118 mg/dL), and a protein level of 123 mg/dL. Empiric intravenous immunoglobulin treatment was started, with a plan to administer it for 5 days.
Thoracic MRI showed an abnormal cord signal involving the posterior columns at the T11-T12 level (Figure 1).
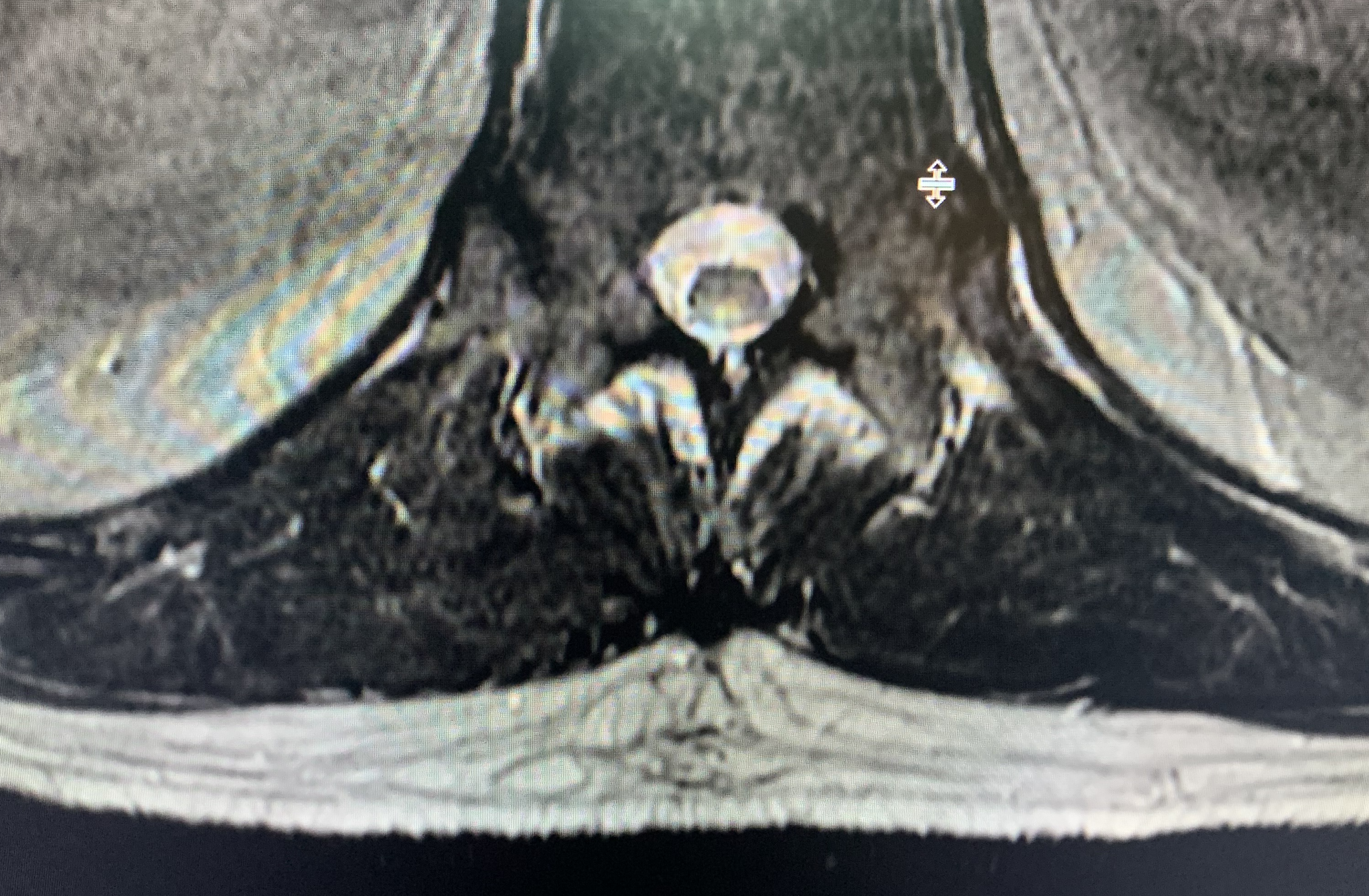
Figure 1. Thoracic MRI showed an abnormal cord signal involving the posterior columns at the T11-T12 level.
The patient’s vitamin B12 level was found to be low at 178 pg/mL (reference range, 211-911 pg/mL). Upon further review of the patient’s history, he was emotional and tearful and admitted to using approximately 24 “whippits”—pressurized cartridges of nitrous oxide (N2O) used to charge whipped cream dispensers (Figure 2)—daily for the past several months in an effort to cut down on his daily marijuana use.

Figure 2. N2O whippits (DMTrott/Wikimedia Commons/CC-BY-SA-4.0).
More tests were ordered. The folate level was normal at 19.7 ng/mL, the homocysteine level was elevated at 86.5 µmol/L (reference range, 3.7-13.9 µmol/L), and the methylmalonic acid (MMA) level was severely elevated at 3154 nmol/L (reference range, 87-318 nmol/L). A peripheral blood smear revealed normocytic anemia and neutropenia with many hypersegmented neutrophils showing 4 to 6 lobes. Results of rapid plasma reagin, antinuclear antibodies, HIV, and antiphospholipid antibodies tests were all negative. Urine drug screen results were negative. No oligoclonal bands were detected in the CSF.
The patient’s presentation was consistent with N2O use–induced vitamin B12 deficiency with subacute combined degeneration (SCD) of the spinal cord.
He was started on parenteral vitamin B12, 1000 µg intramuscularly for 3 consecutive days followed by 1000 µg intramuscularly once a week for 4 weeks. He was concurrently started on oral vitamin B12, 2000 µg daily. He underwent acute rehabilitation and had resolution of his paresthesias, ataxia, and ambulatory dysfunction within the next 4 to 6 weeks, after which he reported walking up to 4 miles a day. Nevertheless, he has had persistent neurogenic bladder and has had to self-catheterize.
Discussion. N2O, commonly known as laughing gas, has been used as an inhalational anesthetic agent, with its most common use in dental and obstetric practices. Its use as a recreational drug has been rising in the United States and Europe. According to the Global Drug Survey 2019, N2O is the 10th most commonly abused substance globally (after excluding alcohol and tobacco/nicotine products.1 An earlier Global Drug Survey reported that 91% of users used N2O once or less per month, with 4% of users reporting symptoms consistent with nerve damage in the following year.2
N2O cartridges or cylinders that are used recreationally are typically designed for whipped cream and are referred to as whippits. They are available in supermarkets, smoke shops, and online. Their contents are either inhaled directly or transferred into a balloon before inhalation.3 Inhalation causes euphoria, giggling, giddiness, and sometimes hallucinations within 20 seconds and lasting few minutes.3,4
The most devastating consequence of N2O use is SCD of the spinal cord secondary to vitamin B12 deficiency. N2O acts by direct depletion of vitamin B12 by irreversibly binding to cobalt ions of vitamin B12 and inactivating it.5 As a result, vitamin B12 is not available as a coenzyme to convert homocysteine to methionine (increasing the homocysteine level) or methylmalonyl-CoA to succinyl-CoA (increasing the MMA level). This interrupts methylation of myelin proteins, leading to instability of myelin sheaths and axonal loss.5,6 Both the central and peripheral nervous systems may be affected. The cervical spinal cord has the highest density of myelinated fibers in the fasciculus gracilis and is particularly susceptible.7 The thoracic spinal cord is also vulnerable. N2O also can cause cognitive behavioral changes by activation of γ-aminobutyric acid and inhibition of N-methyl-d-aspartate pathways in addition to release of endogenous opioids.8,9
The key diagnostic features of SCD are early involvement of the posterior and lateral columns of the spinal cord presenting as paresthesias and proprioceptive loss without motor or autonomic deficits.10 Markers of spinal cord inflammation include an elevated CSF protein level with absent oligoclonal bands/immunoglobulin G index and absent CSF pleocytosis.10 The characteristic MRI findings are symmetrical T2 hyperintensity within the posterior columns, with or without lateral column involvement.11 This produces the classic inverted V sign on axial T2-weighted images.12
In a systematic review of 91 cases with N2O use, vitamin B12 levels were below normal in 33 cases, low‐normal in 12 cases, and normal or elevated in 16 cases.5 Thus, the serum vitamin B12 level is not a measure of total body vitamin B12 and may remain normal despite low vitamin B12 tissue reserves.13,14 Therefore, functional vitamin B12 deficiency should be assessed by measuring homocysteine and MMA levels.4,6 Nearly all patients with SCD have an MMA level of greater than 500 nmol/L.13
A history of repeated N2O use or a single use in a person with subclinical vitamin B12 deficiency can lead to SCD.15 The average reported consumption of N2O users is 10 whippits per consumption, with 80% of users only using once per year.15,16 In a case series of 6 patients published by anesthesiologists, N2O anesthesia for as little as 90 minutes during a single surgery caused severe neurologic deficits, with time of onset varying from 2.5 to 6 weeks postoperatively.9
The mainstay of treatment is immediate withdrawal of N2O exposure and prompt vitamin B12 supplementation.17 Vitamin B12 therapy can be administered orally or intramuscularly.18 The usual recommended dose is 1000 to 2000 µg daily.18
The prognosis is unclear. In a retrospective review of data from 57 patients with SCD, one group of authors noted clinical resolution in only 8 patients with vitamin B12 therapy.19 In the remaining 49 patients, vitamin B12 stopped progression and improved symptoms but did not lead to complete resolution of neurologic deficits.19 Another group of researchers, however, noted full recovery of muscle power in all 9 adolescents (average age, 17.7 years) with N2O-induced SCD in their study, although 5 of the patients had persistent sensory deficits.20
REFERENCES:
- Global Drug Survey 2019. GDS2019 Key Findings Report. May 16, 2019. Accessed May 19, 2020. https://issuu.com/globaldrugsurvey/docs/gds2019_key_findings_report_may_16_
- Global Drug Survey 2016. The Global Drug Survey 2016 findings. Accessed May 19, 2020. https://www.globaldrugsurvey.com/past-findings/the-global-drug-survey-2016-findings/
- van Amsterdam J, Nabben T, van den Brink W. Recreational nitrous oxide use: prevalence and risks. Regul Toxicol Pharmacol. 2015;73(3):790‐796. doi:10.1016/j.yrtph.2015.10.017
- Keddie S, Adams A, Kelso ARC, et al. No laughing matter: subacute degeneration of the spinal cord due to nitrous oxide inhalation. J Neurol. 2018;265(5):1089‐1095. doi:10.1007/s00415-018-8801-3
- Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358‐369. doi:10.1111/ajad.12372
- Thompson AG, Leite MI, Lunn MP, Bennett DLH. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15(3):207‐209. doi:10.1136/practneurol-2014-001071
- Ohnishi A, O’Brien PC, Okazaki H, Dyck PJ. Morphometry of myelinated fibers of fasciculus gracilis of man. J Neurol Sci. 1976;27(2):163‐172. doi:10.1016/0022-510x(76)90058-7
- Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9‐18. doi:10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2
- Hadzic A, Glab K, Sanborn KV, Thys DM. Severe neurologic deficit after nitrous oxide anesthesia. Anesthesiology. 1995;83(4):863‐866. Accessed May 8, 2020. https://anesthesiology.pubs.asahq.org/article.aspx?articleid=1949600
- Goldish D, Massagli TL. Subacute progressive myelopathy: transverse myelitis or subacute combined degeneration? A case report. PM R. 2018;10(3):320‐324. doi:10.1016/j.pmrj.2017.07.076
- Ravina B, Loevner LA, Bank W. MR findings in subacute combined degeneration of the spinal cord: a case of reversible cervical myelopathy. AJR Am J Roentgenol. 2000;174(3):863‐865. doi:10.2214/ajr.174.3.1740863
- Narra R, Mandapalli A, Jukuri N, Guddanti P. “Inverted V sign” in sub-acute combined degeneration of cord. J Clin Diagn Res. 2015;9(5):TJ01. doi:10.7860/JCDR/2015/14028.5889
- Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149‐160. doi:10.1056/NEJMcp1113996
- Ropper A, Samuels M, Klein J. Diseases of the nervous system caused by nutritional deficiency. In: Ropper AH, Samuels MA, Klein JP, eds. Adams and Victor’s Principles of Neurology. 10th ed. McGraw-Hill; 2014:1161-1185.
- Egan W, Steinberg E, Rose J. Vitamin B12 deficiency-induced neuropathy secondary to prolonged recreational use of nitrous oxide. Am J Emerg Med. 2018;36(9):1717.e1‐1717.e2. doi:10.1016/j.ajem.2018.05.029
- Randhawa G, Bodenham A. The increasing recreational use of nitrous oxide: history revisited. Br J Anaesth. 2016;116(3):321‐324. doi:10.1093/bja/aev297
- Singer MA, Lazaridis C, Nations SP, Wolfe GI. Reversible nitrous oxide–induced myeloneuropathy with pernicious anemia: case report and literature review. Muscle Nerve. 2008;37(1):125‐129. doi:10.1002/mus.20840
- Oh R, Brown DL. Vitamin B12 deficiency. Am Fam Physician. 2003;67(5):979‐986. Accessed May 8, 2020. https://www.aafp.org/afp/2003/0301/p979.html
- Vasconcelos OM, Poehm EH, McCarter RJ, Campbell WW, Quezado ZMN. Potential outcome factors in subacute combined degeneration: review of observational studies. J Gen Intern Med. 2006;21(10):1063‐1068. doi:10.1111/j.1525-1497.2006.00525.x
- Lan S-Y, Kuo C-Y, Chou C-C, et al; PCHAN Study Group. Recreational nitrous oxide abuse related subacute combined degeneration of the spinal cord in adolescents: a case series and literature review. Brain Dev. 2019;41(5):428‐435. doi:10.1016/j.braindev.2018.12.003


