Peer Reviewed
Clear Cell Sarcoma in the Upper Abdomen
AUTHORS:
Rachel E. Macey1 • Mohammed M. H. Kalan, MD2
AFFILIATIONS:
1Medical Assistant, Washington Institute of Surgery, Chevy Chase, Maryland
2Surgeon in Chief, Washington Institute of Surgery, Chevy Chase, Maryland
CITATION:
Macey RE, Kalan MMH. Clear cell sarcoma in the upper abdomen. Consultant. 2022;62(2):e20-e22. doi:10.25270/con.2021.04.00018
Received December 28, 2020. Accepted February 5, 2021. Published online April 30, 2021.
DISCLOSURES:
The authors report no relevant financial relationships.
CORRESPONDENCE:
Rachel E. Macey, Washington Institute of Surgery, 5530 Wisconsin Avenue #1450, Chevy Chase, MD 20815 (washingtoninstituteofsurgery1@gmail.com)
A 22-year-old man with morbid obesity presented to our clinic with a painless lump in his upper abdominal wall that had grown by 30% to 40% from October 2019 to January 2020.
Results of an abdominal examination revealed a deep-seated, firm to hard mass with indistinct margins that appeared to be arising from or firmly attached to the anterior abdominal wall muscle. Results of an ultrasonography scan suggested a probable cystic mass in the subcutaneous tissues of uncertain etiology (Figures 1 and 2).

Figure 1. An ultrasonography scan of the abdomen revealed a mass to the right of the midline in transverse orientation that was 1.96 cm deep.

Figure 2. An ultrasonography scan of the abdomen revealed a mass to the right of the midline in longitudinal orientation, measuring 3.04 × 1.65 cm.
A computed tomography (CT) scan was then performed for further evaluation, results of which confirmed the presence of a mass measuring approximately 2.4 cm in diameter. The characteristics were suggestive of a possible desmoid tumor arising from the right rectus muscle and protruding into the subcutaneous fat (Figures 3-5). A wide resection of the anterior abdominal wall mass with primary abdominal wall reconstruction was performed. During the procedure, the mass appeared to be arising from either the anterior rectus aponeurosis or the rectus muscle, measuring approximately 3.0 to 3.5 cm at its widest diameter.

Figure 3. A computed tomography scan of the abdomen and pelvis in the coronal plane shows the tumor’s location.

Figure 4. A computed tomography scan of the abdomen and pelvis in the transverse plane suggested a possible desmoid tumor arising from the right rectus muscle and protruding into the subcutaneous fat.
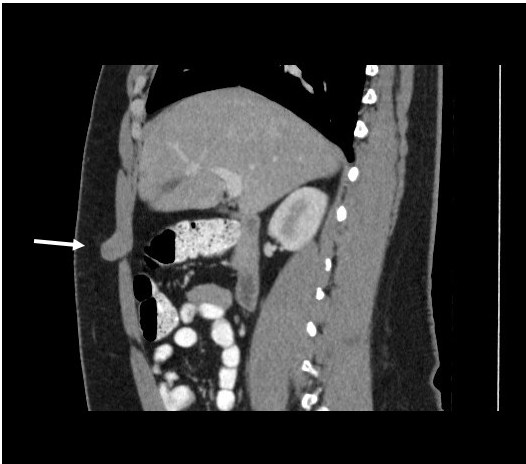
Figure 5. A computed tomography scan of the abdomen and pelvis in the sagittal plane suggested a possible desmoid tumor arising from the right rectus muscle and protruding into the subcutaneous fat.
The mass was excised completely with visibly normal margins of normal tissue on all sides. Pathology test results revealed that the mass was an intramuscular clear cell sarcoma, measuring 3.5 cm with associated necrosis. The neoplastic cells were positive for S100 and HMB-45 and negative for CD34 and Melan-A. The margin of resection was 0.1 mm from the inferior margin and 0.4 mm from the superficial margin.
Treatment and management. A second wider resection was recommended as the first treatment option, but the patient denied further surgery at that time. He was then offered repeat imaging surveillance as an alternative treatment option, as well as a consultation with a medical oncologist. In line with our first recommendation, the patient received 2 additional recommendations from medical oncologists for further surgical excision of the area given the close inferior and superficial margins. The patient has also consulted a plastic surgeon to assist in the abdominal wall reconstruction during the reexcision because of a history of morbid obesity.
The patient has since undergone a postoperative positron emission tomography scan, results of which revealed nodular areas of fluoro-deoxyglucose (FDG) uptake locally with a standardized uptake value (SUV) max of 4.1. This could be attributed to postoperative changes or residual tumor tissue. There were no findings of distant metastases. The patient has since undergone a wide local reexcision, as well as sentinel lymph node biopsies of the left and right axillae with wound closure. He will be followed with long-term imaging surveillance.
Discussion. Clear cell sarcoma was first described by Franz Enzinger in 1965.1 Clear cell sarcoma is a rare malignant tumor that involves the tendons, fascia, and aponeuroses and is most common among adolescents and young adults.1-6 These tumors typically present in White individuals with no tendency toward either gender.3 Tumors frequently occur in the distal and proximal extremities. A majority of cases present in the foot and ankle and are uncommonly found in the trunk.3,5,6 Clear cell sarcoma is associated with a high rate of local recurrence, as well as lymph node and distal metastases.2,4,6 These tumors account for less than 1% of all soft tissue tumors and are often small (< 5 cm), deep tissue lesions that lack cutaneous invasion.5,7 They typically present as slow-growing masses that are often painless with occasional tenderness, which can delay diagnosis and treatment.2-4,8 Diagnosis of clear cell sarcoma is challenging because of its rarity3, no differential characteristic findings appear on imaging, and pathologic examination is needed to establish a confirmed diagnosis.
CT and magnetic resonance imaging (MRI) scans are the standard diagnostic tools, and results of the scans can be used to assess the characteristics and depth of the lesion. CT and MRI scans often show clear cell sarcoma as a distinct, benign-appearing, and homogenous mass.5 In our case, the CT scan revealed the presence of a well-defined, solid mass arising from the anterior abdominal wall. A possible differential diagnosis of a desmoid tumor was entertained by the radiologist. Clear cell sarcoma can also be diagnosed by fine-needle aspiration biopsy instead of incisional biopsy, but sufficient material and immunohistochemical studies are needed.9
On immunohistochemical examination, the tumor cells of clear cell sarcoma typically express the S100 protein.2,3 Most of them also express antigens associated with melanin synthesis such as HMB-45, melanin-A, and Mel-Cam.2,3 In our case, the tumor cells were positive for S100 and HMB-45 and negative for CD34 and melanin-A. One difficulty in diagnosing clear cell sarcoma is that malignant melanoma and clear cell sarcoma display similar immunohistochemical features of strong S100 protein expression and expression of HMB-45 and melanin-A in addition to similar morphology under microscopic and ultrasonography imaging.2,4,5
On microscopic examination, clear cell sarcoma is often described as a dispersed sheet-like pattern of full spindle-shaped cells with a fascicular-like or nested growth of fusiform cells.3,8 The cells also have central ovoid nuclei that are low in mitotic activity and surrounded by clear eosinophilic cytoplasm, hence the name “clear cell sarcoma.”3 Malignant melanoma has similar morphological features, such as round nuclei, clear cytoplasm, and fusiform to polygonal cell shape.8 Cytogenically, a reciprocal translocation can be used to help differentiate clear cell sarcoma from malignant melanoma.3,7,8 Other differential diagnoses include clear cell myelomonocytic tumors, clear cell basal cell carcinoma, dermal melanocytic tumors, balloon cell nevus or melanoma, malignant peripheral nerve sheath tumors, and synovial sarcoma.3,7
Wide local resection of the tumor is the most effective treatment for clear cell sarcoma.10 Chemotherapy and radiotherapy are reserved for patients with metastatic disease, but their roles have been debated.2,3,5-8,11 Our patient was not recommended to undergo chemotherapy or radiotherapy treatment in favor of a second wide local surgical excision. It is necessary for patients with clear cell sarcoma to follow up regularly and undergo continuous surveillance and imaging because of the high risk of recurrence3,5 and tendency to metastasize.3 Early diagnosis and prompt wide resection of a clear cell sarcoma is essential for favorable outcomes2,7 and prevention of metastasis.3
Clear cell sarcoma has multiple suggested prognostic factors, including tumor size, mitotic index, and surgical margins.3-5 Tumor size larger than 5 cm, presence of necrosis, metastasis, and recurrence are often associated with a poor prognosis.2,3,6 According to current literature, tumor size is the best prognostic factor to predict the behavior of the tumor.6,7 Tumors that are smaller than 5 cm are less likely to recur or metastasize, while tumors greater than 5 cm have a higher propensity to recur or metastasize.5
References
1. Enzinger, FM. Clear‐cell sarcoma of tendons and aponeuroses. An analysis of 21 cases. Cancer. 1965;18:1163-1174. https://doi.org/10.1002/1097-0142(196509)18:9<1163::AID-CNCR2820180916>3.0.CO;2-0
2. Jung SJ, Seung NR, Park EJ, Kim CW, Kim KH, Kim KJ. A case of clear cell sarcoma occurring on the abdomen. Ann Dermatol. 2008;20(1):45-48. https://doi.org/10.5021/ad.2008.20.1.45
3. Albeshri MA, Ashour A. Clear cell sarcoma of the foot in an 18-year-old female. Case Rep Orthop. Published online November 23, 2019. https://doi.org/10.1155/2019/8378106
4. Chen S, Luo P, Yang L, et al. Prognostic analysis of surgically treated clear cell sarcoma: an analysis of a rare tumor from a single center. Int J Clin Oncol. 2019;24(12):1605-1611. https://doi.org/10.1007/s10147-019-01487-x
5. Mavrogenis A, Bianchi G, Stavropoulos N, Papagelopoulos P, Ruggieri P. Clinicopathological features, diagnosis and treatment of clear cell sarcoma/melanoma of soft parts. Hippokratia. 2013;17(4):298-302. https://www.hippokratia.gr/2019/03/04/clinicopathological-features-diagnosis-and-treatment-of-clear-cell-sarco-ma-melanoma-of-soft-parts/
6. Hocar O, Le Cesne A, Berissi S, et al. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 52 cases. Dermatol Res Pract. Published online May 30, 2012. https://doi.org/10.1155/2012/984096
7. Rodríguez-Martín M, Sáez-Rodríguez M, Esquivel B, Gonzáalez RS, Cabrera AN, Herrera AM. Clear cell sarcoma: a case mimicking primary cutaneous malignant melanoma. Indian J Dermatol. 2009;54(2):168-172. https://doi.org/10.4103/0019-5154.53193
8. Juel J, Ibrahim RM. A case of clear cell sarcoma - a rare malignancy. Int J Surg Case Rep. 2017;36:151-154. https://doi.org/10.1016/j.ijscr.2017.05.034
9. Tong TR, Chow TC, Chan OW, et al. Clear-cell sarcoma diagnosis by fine-needle aspiration: cytologic, histologic, and ultrastructural features; potential pitfalls; and literature review. Diagn Cytopathol. 2002;26(3):174-180. https://doi.org/10.1002/dc.10081
10. National Comprehensive Cancer Network. Soft Tissue Sarcoma. Accessed February 1, 2021. https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
11. Czarnecka AM, Sobczuk P, Zdzienicki M, Spałek M, Dudzisz-Śledź M, Rutkowski P. Clear cell sarcoma. Oncol Clin Prac. 2019;14(6):354-363. doi:10.5603/ocp.2018.0049


