Peer Reviewed
Tracheal Stenosis After Prolonged Invasive Mechanical Ventilation: An Emerging and Elusive Complication of COVID-19
AUTHORS:
Joseph P. Flynn, DO1 • Jaspal Singh, MD2
AFFILIATIONS:
1Resident Physician, Internal Medicine Department, Atrium Health, Charlotte, North Carolina
2Medical Director, Innovation and Quality Improvement for Pulmonary Oncology, Atrium Health, Charlotte, North Carolina
CITATION:
Flynn JP, Singh J. Tracheal stenosis after prolonged invasive mechanical ventilation: an emerging and elusive complication of COVID-19. Consultant. 2022;62(8):e1-e4. doi:10.25270/con.2021.12.00006
Received November 3, 2021. Accepted November 29, 2021. Published online December 20, 2021.
DISCLOSURES:
The authors report no relevant financial relationships.
CORRESPONDENCE:
Joseph P. Flynn, DO, Atrium Health, 1237 Harding Place, Suite 3200, Charlotte, NC 28204 (Joseph.Flynn@atriumhealth.org)
Post-intubation tracheal stenosis (PITS) is a rare but well-known complication of prolonged mechanical ventilation. Tracheal ischemia caused by prolonged endotracheal intubation is thought to be the primary mechanism of injury leading to scar formation and subsequent tracheal stenosis.1,2 Research published before the COVID-19 pandemic estimated the incidence of PITS to be 4.9 cases per million per year in the United Kingdom,3 but further research is needed to better understand the incidence of PITS during the COVID-19 pandemic.2 According to Shenoy and Nileshwar, tracheal stenosis can be classified by degree of luminal obstruction ranging from grade 1, less than 50% obstruction, to grade 4, no detectable lumen.3 Multiple modalities exist for diagnosing PITS, including chest computed tomography (CT) scanning, pulmonary function tests (flow volume loops with fixed airway obstruction), and bronchoscopy.4 However, given the rarity of PITS, patients will often experience a delay in diagnosis, as symptoms can mimic other more common pulmonary ailments (eg, chronic obstructive pulmonary disease [COPD], asthma, vocal cord dysfunction).1 A delay in diagnosis of this rare complication can lead to poor outcomes and further strain the already over-burdened medical system.
In the wake of the COVID-19 pandemic, a significant increase in the need for mechanical ventilation and increased frequency of prolonged times on mechanical ventilators has been reported.5 It has been observed that patients with COVID-19 had a median duration of ventilation of 17 days and often required high positive end-expiratory pressure through an endotracheal tube.5 Before COVID-19, the risk of developing PITS and overall mortality was lowered with early tracheostomy (about 7-14 days from orotracheal intubation) compared with long-term orotracheal tube management.1 During the COVID-19 pandemic, tracheostomy was delayed because of a variety of issues, including high oxygenation requirements in many patients, the high risk of decannulation in patients requiring prone positioning, and necessity of viral clearance prior to procedure in many hospital systems.
Unfortunately, these barriers have led to patients being intubated for significantly prolonged periods. In our 2 cases outlined below, we observed PITS develop despite relatively reasonable durations of endotracheal intubations. We, and others, have hypothesized that given the increased number of long-term intubations with delayed tracheostomy in critically ill patients, there is a high probability that we will experience a rise in tracheal complications including tracheal stenosis.1,2,6-8 We also hypothesize that many cases of PITS related to COVID-19 will be delayed in diagnosis or missed because of the overlap of symptoms with those of COPD and other recognized respiratory effects of prolonged COVID-19. We, therefore, call attention to PITS out of concern for the great potential of this diagnosis being missed in patients with COVID-19.
CASE 1
A 72-year-old Black woman presented to our emergency department (ED) in August 2020 with worsening shortness of breath, fever, ageusia, and anosmia. She had a medical history significant for polymyositis, autoimmune hepatitis, and obesity with a body mass index of 32 kg/m2.
COVID-19 had been diagnosed via antigen test 8 days prior to her presenting to our ED. On presentation to our ED, she had hypoxemia with an oxygen saturation of 78% on room air. A chest radiography scan conducted at this time showed moderately severe bilateral peripheral alveolar infiltrates consistent with SARS-CoV-2 pneumonia (Figure 1). The patient was initiated on a low dose (6 mg daily) of dexamethasone and remdesivir and admitted to the hospital for supportive care including supplemental oxygen.

Figure 1. A chest radiography scan showed moderately severe bilateral peripheral alveolar infiltrates consistent with SARS-CoV-2 pneumonia.
Initial inflammatory markers indicated likely severe disease, with a C-reactive protein level of 24.7 mg/dL, a ferritin level of 2053 ng/mL, a lactate dehydrogenase level of 469 U/L, and an interleukin-6 level of 203.8 pg/mL. Her hypoxemia quickly worsened, and she underwent endotracheal intubation on hospital day 2. The patient remained intubated and mechanically ventilated for 11 days with intermittent prone positioning.
Convalescent plasma was added to her treatment regimen. She was successfully extubated on hospital day 15 to noninvasive positive pressure ventilation and subsequently titrated over 5 days to 2 L of oxygen per minute via nasal cannula by the time of discharge. The patient was noted to have post-intubation stridor at the time of discharge and was discharged home with a continued course of oral prednisone.
She was seen by an outpatient internal medicine physician approximately 1 month after discharge for concerns of continued shortness of breath, dyspnea on exertion, and reported oxygen desaturations to 80% on room air. Findings from a physical examination conducted at that time were significant for an audible stridor.
The patient received treatment for presumed bronchospasm with nebulized bronchodilators and inhaled corticosteroids. Given the persistence of her symptoms during follow-up approximately 1 month later, the patient was scheduled for a CT angiography of the chest to evaluate for pulmonary embolism or other intrathoracic pathology. The results showed no evidence of pulmonary embolism but indicated focal narrowing of the midthoracic trachea approximately 5 cm below the glottis (Figure 2). The residual lumen diameter at the greatest degree of narrowing was approximately 3 mm in the anteroposterior dimension and 7 mm in the transverse dimension.


Figure 2. An axial view (A) and sagittal view (B) of the CT angiography of the chest showed focal narrowing of the midthoracic trachea approximately 5 cm below the glottis.
The patient was referred to an outpatient pulmonologist who conducted a bronchoscopy, results of which confirmed tracheal stenosis approximately 67 days following extubation (Figure 3). The patient then underwent a 4-cm × 12-mm balloon dilation and a 16-mm × 40-mm endoluminal tracheal stent placement, which improved her symptoms. Her course was complicated by a stent infection with Pseudomonas aeruginosa, as well as stent migration and restenosis upon removal of the stent. Serial dilation and repeat stents were required over the following months until she transferred to a tertiary center in March 2021 with plans for tracheal surgery.

Figure 3. Results of a bronchoscopy confirmed tracheal stenosis approximately 67 days following extubation.
CASE 2
A 64-year-old White woman presented to an outside hospital in October 2020 with progressive shortness of breath and fevers in the setting of known COVID-19 infection. She had a medical history of poorly controlled type 2 diabetes and obesity with a body mass index of 32 kg/m2.
On arrival to the ED, the patient had hypoxia with an oxygen saturation of 80% and was admitted to the hospital for further evaluation. She was emergently intubated and escalated to the intensive care unit following brief asystole and cardiac arrest with return of spontaneous circulation achieved after 2 minutes.
During her initial hospitalization, the patient was given dexamethasone, remdesivir, and convalescent plasma. She was successfully extubated following 13 days of mechanical ventilation with intermittent proning. Her hospital course was complicated by pneumonia caused by Proteus mirabilis and bacteremia caused by Klebsiella pneumoniae. The patient was discharged to an acute rehabilitation facility after about 2 months in the hospital, at which time she still required 3 L of oxygen via nasal cannula.
She then presented to our ED in January 2021 with worsening shortness of breath and work of breathing along with sensation of phlegm stuck in her throat. The patient was admitted to the hospital for a presumed COPD exacerbation. A CT angiogram did not show evidence of pulmonary embolism but showed moderate emphysema.
An otolaryngologist was consulted and conducted a nasal endoscopy and pharyngolaryngoscopy, results of which did not show laryngeal edema or subglottic stenosis. A psychiatrist was consulted for evaluation of anxiety’s role in the current presentation, and the patient was initiated on as-needed quetiapine. A bronchoscopy was deferred by the consultant pulmonologist, and the patient was discharged home with plans for outpatient pulmonary function testing.
In February 2021, the patient was readmitted to the hospital with acute hypoxic respiratory failure requiring bilevel positive airway pressure. A pulmonologist was consulted and conducted inpatient pulmonary function testing, results of which indicated fixed obstructive physiology with a forced expiratory volume in 1 second of 25% and an FEF50/FIF50 ratio of 1.1 (Figure 4).
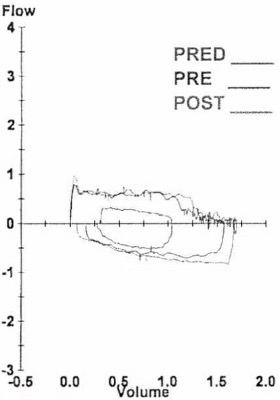
Figure 4. Results of pulmonary function testing conducted by a pulmonologist showed a forced expiratory volume in 1 second of 25% and an FEF50/FIF50 ratio of 1.1.
A follow-up CT scan of the chest indicated severe tracheal stenosis (Figure 5). Because of the significance of the stenosis, the patient developed acute respiratory failure with stridor. This required emergent intubation with a 6.0-mm endotracheal tube placed above the stenosis because of the inability to pass the tube through the stenosis with a bronchoscope (Figure 6). The patient then underwent cannulation for venovenous (VV) extracorporeal membrane oxygenation (ECMO) with subsequent bronchoscopy (Figure 7) and emergent 4-cm × 15-mm balloon dilation.


Figure 5. A follow-up CT scan of the chest indicated severe tracheal stenosis in the axial view (A) and sagittal view (B).

Figure 6. A 6.0-mm endotracheal tube was placed above the stenosis because of the inability to pass the tube through the stenosis with a bronchoscope.

Figure 7. Cannulation for VV ECMO with subsequent bronchoscopy and emergent 4-cm × 15-mm balloon dilation were then performed.
She had rapid improvement in her respiratory status, allowing for extubation at 24 hours post-dilation and decannulation from VV ECMO at 48 hours post-dilation. Her course was further complicated by multiple readmissions because of stent infections and the need for several more balloon dilations and stent placements.
DISCUSSION
In the 2 cases described herein, we report on a rare but severe complication of prolonged mechanical ventilation among a subset of patients with severe acute respiratory distress syndrome (ARDS) caused by SARS-CoV-2. Based on our literature review, the incidence of PITS in this patient population is currently unknown, and additional retrospective research is needed. Fiacchini and colleagues evaluated the incidence of full-thickness tracheal lesions and tracheoesophageal fistulas in patients with COVID-19 after prolonged mechanical ventilation.9 They found a statistically significant increase (nearly 50%) in these tracheal complications compared with a control group and discussed multiple mechanisms specifically related to COVID-19 physiology. They also identified treatment sequela that may explain the greater incidence of tracheal complications, including frequent pronation, which increases cuff pressure on the tracheal walls and high viral replication in the tracheal epithelium which in turn weakens the mucosa.9 Although these findings do not directly address the incidence of PITS in patients with COVID-19–associated ARDS, it could be theorized that a similar increase in the incidence of PITS will be seen over the course of the COVID-19 pandemic in later reviews.
With the potential for increased incidence in PITS and other tracheal complications among patients with COVID-19 requiring prolonged mechanical ventilation, health care providers should be aware of possible changes in management to prevent or diagnose these possible complications. Dr Piazza and colleagues specifically identified possible measures to prevent tracheal stenosis in patients with COVID-19–associated ARDS.1 Among the measures described were to (1) predict difficult airways and designate the most appropriate clinician to manage the case, (2) use correct-sized endotracheal tubes and monitor cuff pressure (between 20-30 mmHg) to prevent mucosal ischemia, and (3) timely transition to tracheostomy to avoid prolonged endotracheal intubation.1
PITS does not typically manifest during the initial intensive care unit hospitalization and often is diagnosed in the weeks to months following discharge with acute presentations for dyspnea and stridor. Follow-up with an otolaryngologist or appropriate airway specialist for patients with COVID-19 and prolonged mechanical ventilation is recommended.1 However, given the significant increase of patients with PITS, it is unlikely that this resource will be equally available among all socioeconomic classes. Increased awareness is needed among all health care providers to accurately diagnose PITS, as this can often be misdiagnosed as asthma or other pulmonary conditions. Appropriate, early diagnosis and prompt referral to clinicians with specific expertise will help limit further complications.
References
1. Piazza C, Filauro M, Dikkers FG, et al. Long-term intubation and high rate of tracheostomy in COVID-19 patients might determine an unprecedented increase of airway stenoses: a call to action from the European Laryngological Society. Eur Arch Otorhinolaryngol. 2021;278(1):1-7. https://doi.org/10.1007/s00405-020-06112-6
2. Fiacchini G, Tricò D, Ribechini A, et al. Evaluation of the incidence and potential mechanisms of tracheal complications in patients with COVID-19. JAMA Otolaryngol Head Neck Surg. 2021;147(1):70-76. https://doi.org/10.1001/jamaoto.2020.4148
3. Shenoy L, Nileshwar A. Postintubation tracheal stenosis: a devastating complication! Indian J Respir Care. 2019;8(2):69-70. doi:10.4103/ijrc.ijrc_18_19
4. Solly WR, O'Connell RJ, Lee HJ, Sterman DH, Haas AR. Diagnosis of idiopathic tracheal stenosis and treatment with papillotome electrocautery and balloon bronchoplasty. Respir Care. 2011;56(10):1617-1620. https://doi.org/10.4187/respcare.01143
5. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. https://doi.org/10.1001/jama.2020.5394
6. Vasanthan R, Sorooshian P, Sri Shanmuganathan V, Al-Hashim M. Laryngotracheal stenosis following intubation and tracheostomy for COVID-19 pneumonia: a case report. J Surg Case Rep. 2021;2021(1):rjaa569. https://doi.org/10.1093/jscr/rjaa569
7. Gervasio CF, Averono G, Robiolio L, et al. Tracheal stenosis after tracheostomy for mechanical ventilation in COVID-19 pneumonia - a report of 2 cases from northern Italy. Am J Case Rep. 2020;21:e926731. https://doi.org/10.12659/ajcr.926731
8. Mattioli F, Marchioni A, Andreani A, Cappiello G, Fermi M, Presutti L. Post-intubation tracheal stenosis in COVID-19 patients. Eur Arch Otorhinolaryngol. 2021;278(3):847-848. https://doi.org/10.1007/s00405-020-06394-w


